PCP-Molarities and chemical concentrations
1/20
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
21 Terms
What is Concentration?
Concentration refers to the ratio of the amount of the ingredient to the amount of product.
What are the different ways concentration can be expressed?
It can be expressed in a number of different ways:
1.Quantity per volume
2.Percentage concentrations
3.Ratios
Parts
What is the Quantity per volume expression and Describe ?
•A quantity per volume expression gives the amount or weight of a drug (either in moles or grams) in a volume of solution.
•It is also referred to as an amount strength.
•For example, a 9 g/L solution of sodium chloride means that 9 grams of sodium chloride are dissolved in 1 litre of solution.
Example calculation - Quantity per volume
500mg= 0.5g
200ml – 0.2 L
0.5/0.2= 2.5g/L

What is percentage concentrations and describe it?
•Percentages are used to express the amount of liquid or solid active ingredient in a defined amount of liquid or solid product.
•There are four commonly used percentage terms:
1.% w/w (percentage weight in weight)
2.% w/v (percentage weight in volume)
3.% v/v (percentage volume in volume)
% v/w (percentage volume in weight)
What is % w/w?
•Weight of a solid active ingredient in 100 g of a solid product.
•For example, 1% w/w denotes that 1 g of active ingredient is contained in 100 g of the final solid product.
How much active ingredient have we got in …
What is % w/v ?
Weight of a solid active ingredient in 100 mL of a liquid product.
For example, a 2% w/v means there is 2 g of drug in 100 mL of the final liquid solution
What is the % v/v ?
•Volume of liquid active ingredient in 100 mL of a liquid product.
•For example, a 30% v/v denotes there is 30 mL of drug in 100 mL of final liquid solution.
What is % v/w?
•Amount of liquid drug in millilitres in 100 g of solid product.
•For example, 40% v/w means there is 40 mL of drug in 100 g of solid product.
Example calculation- using %w/v
1.25g in 100ml
1.25/100 × 540 = 6.75g

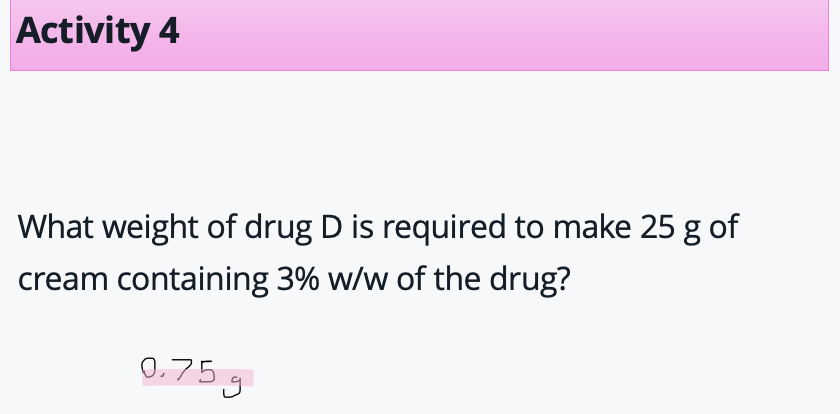
Example calc - % w/w
3g per 100g
find in 1 g then multiply up : 3/100 × 25 = 0.75g
What is a Ratio concentrations and describe them ?
•Ratios are used to express the amount of one pharmaceutical ingredient relative to the amount of another.
•There are two phrases used for ratios that are similar but would yield different amounts…
to (1:10 1 drug 10 parts X ) -total added together
in ( 1 in 10 1 part drug ,9 parts X) -total 10 (stated)

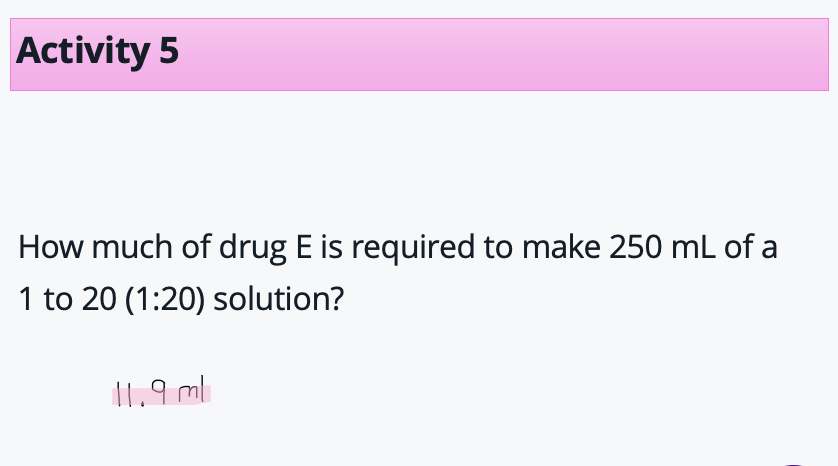
Example calculations- to ratio
1ml of drug in 20ml in solvent
21 ml total
250/21 = 11.9ml
What is parts of concentration (ppm) and describe it ?
•Parts per million (ppm) is used for concentrations where the ratio of active ingredient to product is very small.
•It is equivalent to a ratio of p in 1,000,000.
•1 ppm w/v = 1 g in 1,000,000 ml
Example calculation- ppm
0.45g in 1,000,000ml of drug
450mg in 1,000,000ml
1mg :1000000/450 =
222ml = 2.22l
How to convert between concentrations- ratio general method ?
step 1- write out information
step 2- convert units to g
step 3 - find what is in 1 g
step 4 write in a ratio
EXAMPLE : ratio - concentration conversion calculation
Ratio concentration-
10mg in 5 ml
2. 0.01g in 5ml
3. In 1g:
5/0.01=500ml
4 . RATIO- 1 in 500

How to convert between concentrations - percentage general method?
step 1- write out information
step 2 - convert any units
step 3 - find what is in 100ml
step 4 - write as a percentage
EXAMPLE- percentage ratio calculation
1. 10mg in 5 ml
0.01g per 5ml
3. 100ml – 20x0.01= 0.2 g
4. 0.2%

What is molarity ?
•Molarity is the measure of the number of moles of a substance dissolved in litres of a solution.
What is Molarity equation?
Molarity (mol/L) = number of moles / volume (L)