Biochem Exam 3
1/329
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
330 Terms
What is the definition of a lipid? How does this relate to the diversity of lipid structure?
Something hydrophobic, something with low water solubility. There is a lot of things that are not soluble in water ➡ a variety of structures
What is a common feature of lipids? Can you give an example?
Presence of hydrophobic elements
Example: Long hydrophobic tails found in fatty acids, long repeats like those found in isoprene molecules
Why are lipids important? Where can they be found [as in what processes]?
We can store energy in lipids
They can provide insulation from harsh environments
Important component of membranes
Steroid hormones
Pigments
Certain molecules that participate in inflammation processes
Why are lipids good for storing energy? How efficient is this process?
They are good for storing energy because of the aforementioned hydrophobic elements, specifically the fatty acid tails and triacylglycerols. These have a reduced carbon that can be oxidized which would release a lot of energy. This process is pretty efficient - oxidation of fatty acids is more efficient in amt of ATP per carbon than oxidation of carbohydrates because in fatty acid tails carbon is in a more reduced state. Fatty acid tails have a reduced carbon that can be oxidized to release energy
They are also good for storing energy because they can be packed tightly. Comparatively, carbs like glycogen and starch are surrounded by hydration shells (water molecules) which add to space. Lipids have hydrophobic interactions that exclude water and allow for tight packing. Hydrophobic properties of lipids allow for tight packing.
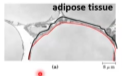
How does adipocyte show that lipids are good for storing energy?
Area within the red line is lipids and it is so tightly packed that it makes up 90% of cell volume. In the periphery is the organelles of the cell ➡ Shows tight packing of lipids!
What’s the problem with lipids being so good at long-term storage?
Anything’s that great at storing things, it’s hard to get those things out of storage. So, it’s hard to transport hydrophobic molecules in blood, so for our daily energy needs we use carbohydrate based storages like glycogen.
Lipids and Environmental Isolation
In polar animals like sea lions and penguins, they have a thick layer of fat that keeps them warm, insulting them from the cold and allowing them to maintain body temp.
Plants in hot climates have a wax to prevent the loss of water.
From one molecule of glucose, we can make how much ATP?
32 molecules
From one molecule of fatty acid (palmitate), we can make how many molecules of ATP?
106
3 Types of Storage Lipids
Fatty acids
Triacylglycerols
Waxes
What is a fatty acid?
The simplest lipid
NEED TO KNOW HOW TO DRAW
Carboxylic head - COOH
Hydrophobic tail
Methyl head
What does it mean if a fatty acid is saturated?
No double bonds!
How do you name this fatty acid?
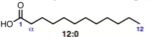
Number of carbons = 12
Number of double bonds = 0
12:0
n-dodecanoic acid
Common name: lauric acid (don’t need to know this)
Fatty acids are synthesized from ___ (1/2/3) carbon molecules known as ______.
2, acetyl
Fatty acids are usually an ____ (odd/even) number of carbons.
even
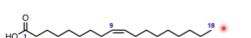
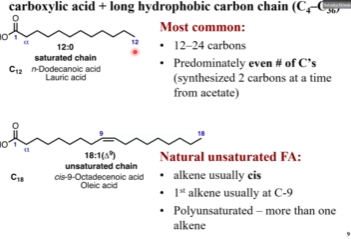
How do we name this fatty acid?
It’s unsaturated, so number of double bonds = 1
Double bond located on the ninth carbon
Amount of carbons is 18
18:1 (Δ9)
cis-9-octadecenoic acid
The 9 is where the double bond is, you start counting from the carboxyl carbon
Common name: oleic acid (don’t need to know this)
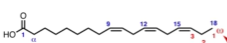
How do you name this bad boy?
18:3 (Δ9,12,15)
What is an omega 3 fatty acid? Can our bodies make omega 3 fatty acids?
It’s a fatty acid where the terminal double bond is always on the third carbon from the end. An example is shown to the left. Our bodies cannot produce this double bond, so we must get it from our diets.

In saturated fatty acids, the longer fatty acid will have _____ (higher/lower) melting temperature. Why?
Higher melting temperature
The tails of fatty acids hydroponically interact with each other to melt, and so, in order to melt these interactions you need to invest more energy.
Longer tail ➡ stronger overall hydrophobic interactions ➡ hard to break these interactions ➡ higher melting temperature
As you chop 2 carbon units, melting temperature changes by about 6-10 Celsius.
In saturated fatty acids, the longer the fatty acid the _____ (more/less) soluble it is. Why?
Less soluble
This is for similar reasons as melting point
Longer tail ➡ stronger overall hydrophobic interactions ➡ hard to break these interactions ➡ lower solubility
It will be more soluble in hydrophobic solvents however.
As you add the first double bond to the fatty acid, does the melting point increase or decrease? Why?
It decreases because it’s the first double bond, and thus the structure of the fatty acid has been distorted and it cannot form nicely organized arrays and does not have the nice hydrophobic interactions to stabilize it and soooo it’s easier to break these interactions and the melting point decreases 😥
Double bond ➡ not pretty structures/weak hydrophobic interactions bc of the distortion ➡ lower melting pt
Why does the introduction of the first double bond do something different than introduction of subsequent double bonds?
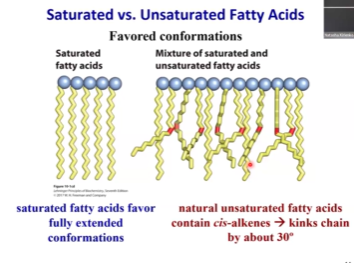
Saturated fatty acids are quite linear and easy to pack. They make a crystalline-like structure that has a large number of hydrophobic interactions stabilizing, resulting in a high melting temperature.
Once we introduce the double bond, it makes the molecule bend. It will introduce a thirty-degree kink in the molecule.
Most unsaturated fatty acids have cis double bonds. Trans fatty acids are very rare. These cis double bonds introduce the kinks btw and distort the structure, making it bend. (rmbr this when drawing fatty acids)
This bending and distortion makes it very hard for these molecules to pack tightly. It is hard for these molecules to have stable, hydrophobic interactions with each other.
If you introduce subsequent double bonds, they will also be cis and introduce additional kinks into the molecule. But, it won’t do anything that dramatic since the ability to make nicely organized arrays has already been distorted 🤷♀ 🤨
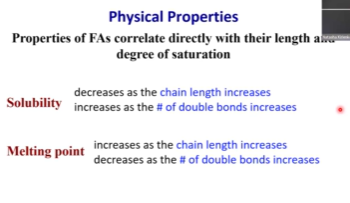
Correlation between properties of fatty acids and their length and degree of saturation
S o l u b i l i t y
DECREASES as chain length INCREASES
INCREASES as number of double bonds INCREASES
M e l t i n g P o i n t
INCREASES as chain length INCREASES
DECREASES as number of double bonds INCREASES
Think about how molecules pack into arrays 😃
The length of a typical fatty acid in the cell is…
18-24 carbons long
Trans fatty acids are very _____ (rarely/commonly) produced naturally.
Rarely
How are trans fatty acids made?
We start with unsaturated fatty acids and add hydrogens to them to convert to saturated fatty acids. But, this hydrogenization could be incomplete.
Under heat, the cis fatty acid can snap into trans fatty acid.
It is the byproduct of heating standard cis-unsaturated fatty acids.
Trans fatty acid conformation is _____ (more/less) stable.
More, so if it switches to a trans fatty acid, it will probably stay that way
What is the issue with trans fatty acids?
Our body still recognizes the double bond, and will put it in the position where they put the unsaturated fatty acids. But, the properties of trans fatty acids are identical to hose of saturated fatty acids since they are quite linear and can pack tightly.
The incorporation of a large proportion of trans fatty acids in the membrane will dramatically decrease membrane fluidity and impede membrane function.
Large amounts of trans fatty acids ➡ decreased membrane function 🙁
This is obvi bad for organismal function
Fatty acids are typically _____ (very/not very) abundant in cells.
Not very
Triacylglycerol - What is their structure like? What makes up the backbone?
Three fatty acids in their structure
Glycerol backbone
One molecule of glycerol + 3 molecules of fatty acids = triacylglycerol
Commonly, there is an unsaturated fatty acid in the middle position
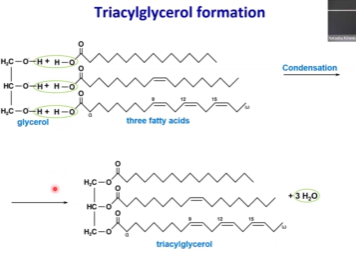
Glycerol Structure
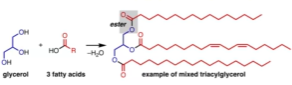
This is an example of a triacylglycerol
😃freebie😃
What are triacylglycerols good for and why?
They are a great source of storage because the carbon is quite saturated and because they can pack tightly.
Waxes - What are they? What can they be used for?
Another type of lipid
Also has long hydrophobic tail that allows for hydrophobic interactions
Cute little polar ester group
Very hydrophobic
They could be used for structural purposes or for plants to prevent water loss. 🌵 🐝
You just need to know they’re a type of lipid
Membrane lipids vs Storage Lipids
Membrane lipids are more polar.
5 Types of Membrane Lipids
Glycerophospholipids
Sphingolipids
Sterols
Galactolipids
Archaeal Tetraether Lipids
Glycerophospholipids - Structure? Comparing it to triacylglycerols, what are the main differences? Do we gotta know how to draw this baby?
Glycerol backbone as in free acyl glycerol
Two fatty acid tails
Phospholipid can be found instead of the third fatty acid that you see in triacylglycerol 😁
We see phosphorylation
The phosphoryl can be further modified by some polar head group, like different types of alcohols or sugars or amino acids
Length of fatty acid is between 16 and lower 20s
Middle position would have unsaturated fatty acid
DO NOT NEED TO KNOW HOW TO DRAW BUT SHOULD KNOW HOW TO ✨ RECOGNIZE ✨ - ARE WE LOOKING AT A TRIACYLGLYCEROL OR A GLYCEROPHOSPHOLIPID 🤔 (if you so phosphoryl that’s def not a triacylglycerol)
Main Points: glycerol back bone, two fatty acid tails, bigger polar head
More polar than triacylglycerol (bc of that fat juicy polar head 😋 )
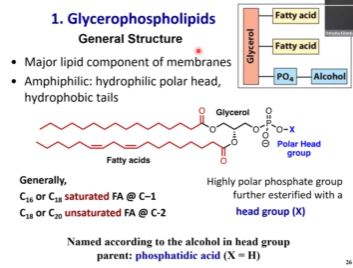
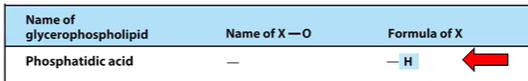
Phosphatidic Acid
It’s just an H
The polar head group is just an H
Simplest possible phospholipid
😇
Phosphatidylcholine Head Group
Very common head group

Head groups for glycerophospholipids _____ (can/cannot) vary greatly in size.
Can
Cardiolipin is giant bc the head grp is another phospholipid and phosphatidic acid is teeny tiny cause it’s just H.
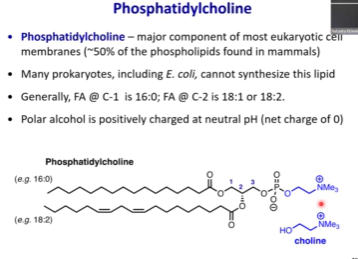
Phosphatidylcholine - How common is it? Charge of phosphoryl group? Charge of choline? Net charge?
Very common - it is found in all mammals.
Negatively charged phosphoryl grp + positively charged choline = net zero charge = neutral lipid
At physiological pH, this will be uncharged .
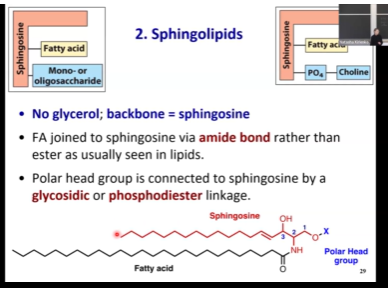
Sphingolipids
Backbone is sphingosine
Only have one fatty acid because sphingosine itself has its own hydrophobic tail
Sphingosine has amide linkage - if you see NH instead of ester linkage, it must be sphingolipid
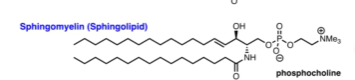
Sphingomyelins - Structure? Where are they found?
Very important in neuron cells
Also has a choline
Ceramide
Also just an H
Very simple head group for sphingolipids

Phosphocholine
The name of the polar head group for sphingomyelin.

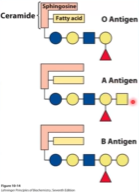
Sphingolipids and Blood Groups - How does this relate to antigens? What about antibodies? 🩸
Erythrocytes always have glycosylated lipids in the membrane and there is always some glycosylation present. The difference would be in the specific terminal sugar. This sugar could be either GalNAc or Galactose.
GalNAc is N-acetylgalactosamine and is usually represented by a square
Galactose (Gal)
The difference between people of different blood types is the absence or presence of terminal sugar. If it is just the core and there is no terminal sugar, that is the O antigen. If the terminal sugar is GalNAc, it is an A antigen. If it is a Galactose, it is a B antigen.
Antigens are present on the glycolipids of blood cells.
Antibodies bind to antigens, so the presence of antibodies in the blood plasma also tells us about blood today. Anti A antibodies will bind to A, anti B will bind to B, and so on and so forth. When antibody binds to antigen, it will coagulate. Blood type O has no antigens and will have both antibodies.
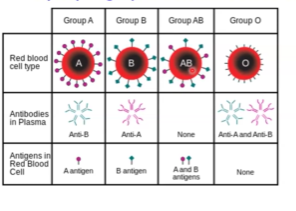
So, let’s think about this logistically: Blood type O has both anti-A and anti-B antibodies because there is no antigens, so it will be fine no matter what.
Blood type B has anti-A antibodies since it has no A antigen so it will be fine.
Blood type A has anti-B antibodies since it has no B antigen, so it will be fine.
Blood type AB will have no antibodies since it has both antigens.
Which blood types have which antigens and which antibodies?
yay group
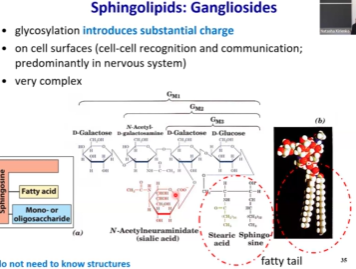
Gangliosides - What’s their problem man?
Have a very unique carbohydrate in them known as N-acetylneuraminidate (sialic acid). This carbohydrate is negatively charged and impacts the glycol- portion of glycolipids and thus impacts cell-cell interactions.
What breaks down lipids? How does this process work?
Lipid breakdown happens in lysosomes. They can fuse with organelle membranes or plasma membranes and start remodeling lipids there and then degrading some types of lipids.
Case Study: Tay-Sach’s Disease
There is a lot of enzymatic steps needed to remove the glycolytic portion of a lipid. You gotta remove one sugar at a time with special enzymes. If one of those enzymes is mutated, the downstream product will accumulate.
Mutation of hexosaminidase A (which helps break down gangliosides) results in Tay-Sach’s Disease (a type of lysosomal storage disorders). In this case, GM3 cannot be further recycled and the lysosomes will only become bigger and bigger and impede cell function. Because gangliosides are very important in neuronal cells, these mutations in their processing will lead to impaired functions of neurons. This can result in blindness, paralysis, etc.
Sterols - Structure?
Not fatty acid based
4 conjugated ring structure
Small polar head group - OH (hydroxyl)
Big hydrophobic portions
Sterols are bulky and thus hard to pack. This also makes it so sterols can easily affect membrane fluidity and permeability.
They thicken plasma membrane
The percentage of sterols in the membrane can reach up to 25%, so they are important constituents of the membrane.
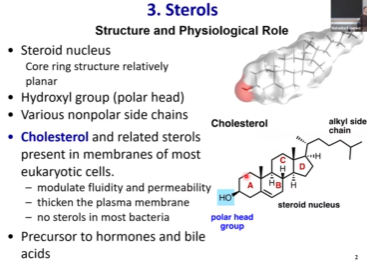
Most common sterol
Cholesterol!
Do bacteria have sterols?
Nope, they have hopanoids (?) which have a five conjugated ring structure. They are analogous to sterols, but are not sterols.
Do plants have cholesterol?
No, they have different phytosterols, like stigmasterol.
Sterols and Hormones - What is the key difference bw the two? What is the relationship bw the two? What do steroid hormones do?
Sterols are precursors to hormones, specifically steroid hormones.
“Many hormones are oxidized derivatives of sterols.”
“Made from cholesterol in gonads and adrenal glands”
Cholesterol has hydrophobic alkyl side chain that steroid hormones do not have.
Steroid hormones are more polar than cholesterol. They can easily travel through the blood after being made in one tissue to get to the target tissue. At the target tissue, they affect cell signaling and developmental processes. Many of the steroid hormones are sex hormones.

If you put multiple lipid molecules in water, what happens? What structures can they form? Is what happens entropically favorable?
Membrane lipids are amphipathic - their tails are hydrophobic and their heads are hydrophilic (and polar).
If there is a number of them in solution, they will orient themselves in such a way that polar heads are next to each other and hydrophobic tails are next to each other. This will maximize the efficiency of these interactions.
When lipids are in aqueous solution, they can form different structures:
Micelles
Bilayers
Vesicles
In all of these structures, lipid tails interact w lipid tails. If lipids interact with each other, then they have hydrophobic interactions that are beneficial, and water molecules can interact with other water molecules. Everyone is happy, all the interactions are beneficial, this is great from an entropic point of view.
The increase of entropy in water is one of the driving force for lipid aggregation in aqueous solution.
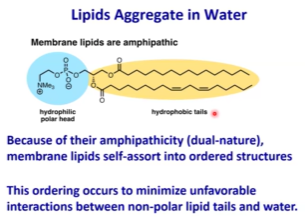
Micelles - Structure? Key factors? Cross section shape?
Simplest type of lipid particle
Only form when cross-section of given lipid is a cone. In order for the cross section to be a cone, the cross section of the head should be wider than the cross section of the tail. When you line up the cones in 3D, it will make a sphere.
The size of the micelles will be partially dependent on the concentrations of lipid in solutions, as well as the length of the lipid tails.
If tails are shorter, diameter of micelle will be shorter
Will be somewhat rare since the lipid needs to have a wider head than tail
If you want to transport anything inside the micelle, it must be hydrophobic
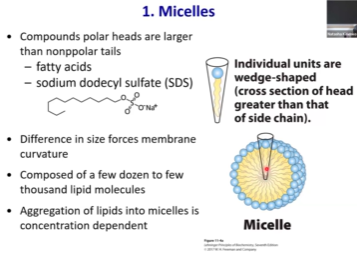
Membrane Bilayer - Function? Structure? Cross section shape?
Consists of two leaflets (e.g. layers) of lipid monolayers
Cross section of the tail is wider in comparison to micelles. The cross section of the tail and the head are similar in size, so the molecules are more straight and cylindrical. If we start packing them together, we’ll get a fence-like structure.
Often involve compounds with >1 lipid tail such as glycerophospholipids and sphingolipids.
If we form a leaflet, the hydrophilic heads on the top and hydrophobic tails on the inside. The hydrophobic tails of the two leaflets interact with each other. Water is excluded, contributing to the favorability of this process.
These make up the membranes of our organelles. We have lots of membranes that allows us to subcompartmentalize different organelles within the cell.
So, we can separate our lysosome that has low pH or nuclear membrane that protects DNA from reactive oxygen species that are abundantly generated in the mitochondria
Membrane allows for differentiation between self and outside
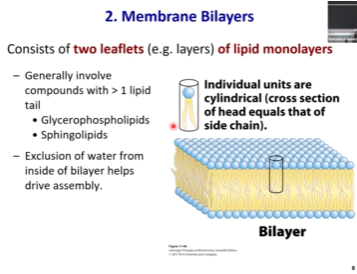
Vesicles - AKA? How do they form? Which parts are hydrophobic/hydrophilic?
Lipid bilayers can spontaneously fold on itself and seal into a spherical vesicles ( ~ physics~ )
You should know that bilayers fold to make liposomes (vesicles) or in extension cells
Once the bilayer folds on itself, eventually, it will make a large sphere known as a liposome (vesicle) that will have an aqueous cavity inside since that were one part of the hydrophilic heads from the bilayer is now located.
The liposome is a great way to deliver hydrophilic drugs because hydrophilic cargo will fit very comfortably inside the vesicle
Vesicles can fuse with cells on their own. There is also protein machinery that will facilitate interaction between the vesicle and the cell.
What are membranes?
They pliable sheets ➡ bilayer
Composed of multiple components
Lipids
Proteins (about 50% of some membranes, but in membranes like the mitochondrial membrane, it’s about 80% protein)
Some membranes have RNA
All cells have a membrane that separates the cell from its surroundings
Very complex!
Why are membranes important?
They define boundaries of the cell
They provide compartmentalization within the cell
Inside our cells, we have a lot of ATP and vitamins and important things. Membranes are semi-permeable. There are transporters within the membrane that allow for transport. Transport allows for the creation of things like proton gradients and supports ATP synthesis.
Signaling: transmit external signals into cells for conversion into response (including nerve signals)
Membrane-membrane interactions with receptors and the molecules that recognize them in different cells help immune surveillance.
What do membranes look like? What determine which state the membrane is in? How does this relate to function?
The lipid bilayer can be in a couple of different states. If we have lots of highly saturated fatty acids, then they will make very strong hydrophobic interactions with each other and make the liquid-ordered state. ➡ Gel-like structure
If we have a large number of unsaturated fatty acids, or we have a high temperature, then we will have a liquid disordered state. Once we have the kink from the unsaturated fatty acids, we can no longer pack the lipids tightly and they will be highly mobile. ➡ Highly fluid state!
Most of our membranes are like this, and this is very important for their functions since it makes it easy to remodel the membrane and enables lateral movement within the membrane.
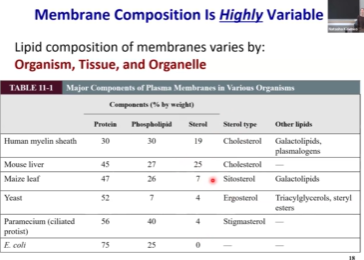
The Variability of Membrane Composition
Protein composition varies between different systems.
30% in myelin sheath
75% in E. Coli
In humans, we have proteins that do different jobs in different organelles and within their membranes. We don’t really need to squish every function of the cell into the PM. But, because bacteria don’t have organelles like the Golgi and the mitochondria, they have to squish every function into the PM, so their protein composition is much higher.
Membrane composition depends on the organism it is coming from.
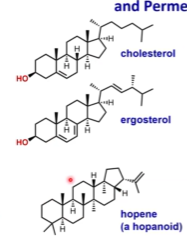
Sterols (and Hopanols) and Membrane Rigidity
Sterols are bulky and make membranes more rigid ➡ You cannot be in liquid state when it’s something that does not pack well together
But, if we decrease the temperature, we will decrease the motion. Lipids like sphingolipids will become less mobile and the membrane overall will become less fluids. Sterols will allow for the membrane to become more fluid.
There are little pores around sterols due to the inability to pack them tightly through which small molecules can cross the membrane ➡ increased permeability
Low temperatures ➡ sterols improve fluidity
High/normal temperatures ➡ sterols increase rigidity
If you want to make the membrane more fluid, what’s the easiest way to do it? What about to decrease fluidity?
Increase fluidity ➡ increase temperature
Decrease fluidity ➡ decrease temperature
What happens if membrane is too fluid?
It will fall apart, forming small vesicles
What happens if membrane is too rigid?
It cannot carry out its functions.
If temperature is high and we want to increase rigidity, what will happen?
We increase number of saturated fatty acids and decrease number of unsaturated fatty acids.
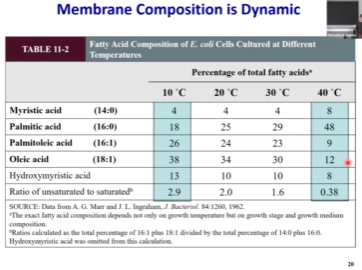
Membrane Composition Difference bw Organelles
Proton gradient in mitochondria is generated across specifically inner mitochondrial membrane but not outer mitochondrial membrane. Cardiolipin (a lipid) is highly enriched in the inner mitochondrial membrane. When looking at the mitochondria, we stain for for the cardiolipin.
Membrane composition can even change between leaflets.
Lateral Diffusion
Movement is very fast and spontaneous
Micron per second
A combination of short-range movements with occasional rare long-range movement.
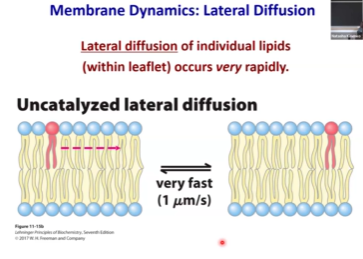
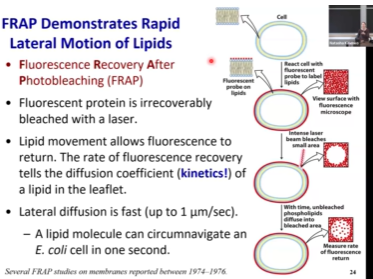
How do we prove that lateral diffusion happens, and that it is very fast?
We use a method known as FRAP - Fluorescence Recovery After Photobleaching
We have a cell where we added fluorescent probe on lipids. The whole cell is covered in the dye and we can see the fluorescence. If we shine a high-energy laser at them (the dye), they will photo bleach and lose their color. We’ll make a spot there the dye is photobleached and there is no fluorescence coming from it. We will very quickly see a signal appearing, and this signal must be the movement of the neighboring molecules in this space.
Using FRAP, we can confirm that lateral movement of lipids within the leaflet of the membranes is very fast.
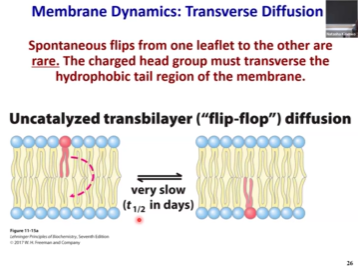
Transverse Diffusion
When a molecule from outer leaflet will go into inner leaflet and vice versa.
In this movement, the hydrophilic head has to go through hydrophobic space which is highly unfavourable.
Delta G of the reaction is zero.
The activation energy barrier is huge.
But, it would literally take days, highly inefficient
This process is called a “flip flop”
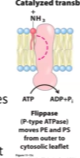
Flippases
Move it from outer leaflet to inner leaflet
Use ATP
Floppase
Moves it from inner leaflet to outer leaflet
Uses ATP
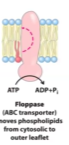
Scramblases
Can move in either direction
Don’t use ATP so they don’t cost any energy, but they can only move molecules down the concentration gradient
Phosphatidylserine and phosphatidylethanolamine are highly enriched in the _____ leaflet (inner/outer). Why?
Inner
This happens because the flippases constantly hydrolyze ATP to move these molecules (PS & PE) into the inner leaflet. Scramblases counteract their actions and move them to the outer leaflet to equalize the gradient, but flippases are much more efficient. That’s why in a normally functioning cell, we have a gradient of PS and PE in the inner leaflet.
What happens to PS and PE when a cell dies? What is this a sign for?
Well, when a cell dies there is no ATP, and so flippases and floppases will no longer work, BUT scramblases will work since they don’t need ATP. And so, scramblases start moving PE and PS down the concentration gradient, which means from the inner leaflet to the outer leaflet. This will result in an increase of PS and PE concentration in the outer leaflet. This is a sign for the neighboring cell that this cell is dead. The neighbor cell can then engluf it and process it - allows for homeostasis yayyy 😄
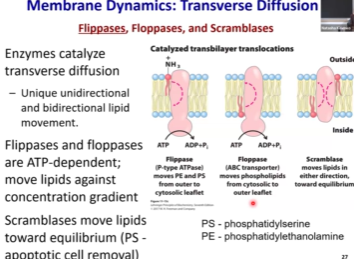
Do proteins move or are they rigid? How do we know this?
We will make a mouse cell and label the mouse membrane proteins with green and human membrane proteins with red. We will then fuse the cells. We will do this via virus to facilitate diffusion since activation energy for fusion is high.
After cells fuse, there will be an initial separation between proteins, but within an hour (actually, less, but around there, also this is a little slower than lipids but still fast), the fluorescent signal will homogenize.
Mouse and human membrane proteins are intermixed ➡ Proteins also move across the membrane
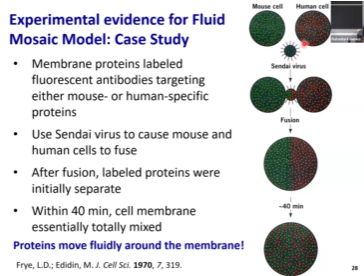
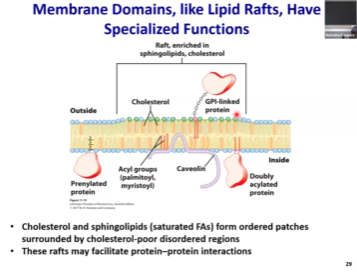
Is the membrane homogenous or does it have subdomains?
The membranes are not homogenous. Certain aspects make certain portions of the membrane less fluid than the rest.
Cholesterol increases membrane rigidity and decreases membrane fluidity!
Membrane rafts enriched in sphingosine which will allow for proteins that need to be together to interact to stay together
Membrane composition is not uniform
We have membrane rafts that are enriched in proteins, cholesterol, and sphingosine, and they will be more stable than the rest of the membrane.
We will have more proteins in the _____ leaflet in comparison to the other one. Why?
Outer leaflet.
This is because there is a lot of cell-cell communication. The proteins in the inner leaflet are mostly for cell signaling processes.
The outer leaflet of the membrane has what characteristics in comparison to the inner leaflet?
Slightly thicker
More ordered
Less fluid
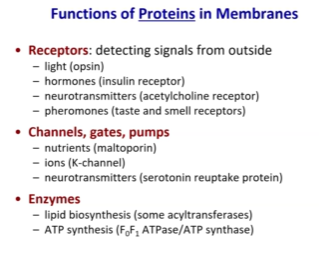
Functions of Proteins in Membranes
Receptors
Sense environment (so in bacteria that are photosynthetic, they can sense light)
They can sense hormones (like insulin receptors)
Neurotransmitter (we need to have for these proper cognitive functions in our brains)
Smell, taste receptors, pheromones
Channels, gates, pumps
Transporters
Nutrients
Ions
Enzymes
Enzymes involved in lipid metabolism will be on the membrane
Lipid biosynthesis (acyltransferase)
Enzymes involved in energy metabolism (ATP Synthase) will also be on the membrane
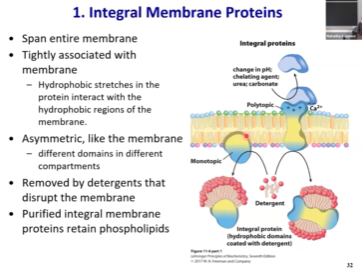
Integral Membrane Proteins - Key features?
“Integrated into the membrane”
Span entire membrane
Tightly associated with membrane
Part of it will be inside the membrane, embedded into the hydrophobic portion
Bc they interact w hydrophobic fatty acid tails, the portion that is inside will also be hydrophobic to make the interactions favorable
Polytopic protein - spans both sides of the membrane
Because they have hydrophobic portions, these portions are tightly interacting with lipids
We cannot purify it away from the lipids, even if we use detergent or sonification
They will have hydrophilic portion that will into cytosol or to the outside and will have hydrophobic portion inside of the membrane
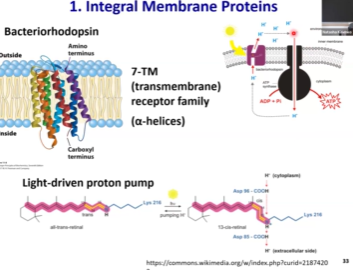
Bacteriorhodopsin - What is it? What’s it do? Who’s it work with? What’s the life hack? 🤔
Example of integral membrane protein!
Changes its conformation in the presence of high energy light
That conformational change in its cofactor retinal results in proton pumping.
In the presence of light, it will be pumping protons from inside of the cell to the outside of the cell to establish proton gradient.
In combination with ATP synthase, which can let protons back into the cell down the gradient (highly favorable), and we can use that energy to generate ATP.
These two proteins and some sunlight can generate endless ATP out of thin air!! WOwww 😯
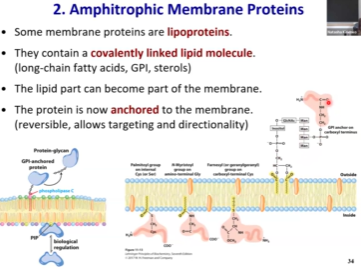
Amphitropic Membrane Proteins - Key Feature? Can ya get it off the membrane?
The protein itself is located in hydrophilic environment (either inside or outside of the cell)
But, they will be anchored in the membrane by some kind of hydrophobic tail.
Will be covalently modified w fatty acid or some other type of lipid that will embed directly into the membrane. This tail can be variable lengths (example: GPI has an anchor that is quite long, the protein can have a large range of motion)
Normally, these proteins are tightly associated with the membrane bc of these lipids portions that are embedded in the membrane. However, these tails usually are cleaved upon regulatory event (like a hormone binds to the receptor that leads to the cleavage of the tail, and the protein will go somewhere else to do its enzymatic function).
If we purify them, the protein itself will not be bound to lipids. They may or may not be associated with the membrane depending on whether signaling is on or off.
Lipoprotein
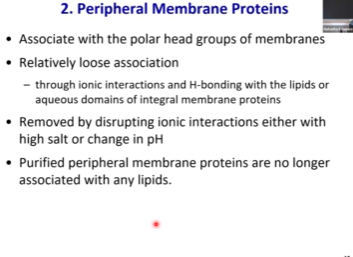
Peripheral Membrane Protein
Those that interact w periphery of the membrane, IE the polar heads
Non covalent interactions (ionic interactions) ➡ loose association
Can be easily interrupted by salt gradient or pH gradient
If we want to purify peripheral protein from the membrane, we just need to increase salt concentration and the protein will fall off
Hydrophobic amino acids
Leucine, isoleucine, valine
Hydrophobic portions of the integral membrane proteins are usually enriched with hydrophobic ________, like ___________.
Amino acids
Leucine, isoleucine, valine
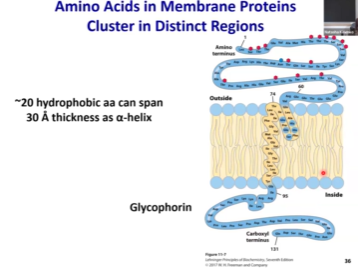
In integral membrane proteins, the parts that are either inside/outside of the cell, would be more ______ (polar/nonpolar).
Polar
Hydrophobic amino acids are usually found ______ the membrane, and if it is polar, it will likely be _______.
inside, outside
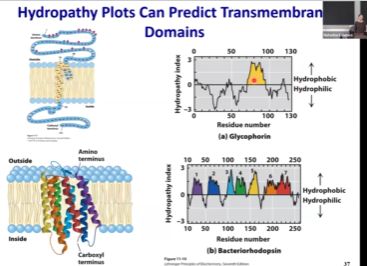
Hydropathy Plots - What do we use it for? What’s a drawback?
Since there is a difference in sequence hydrophobicity, we can predict transmembrane domains by analyzing the sequence.
If amino acid is hydrophilic, it’s hydropathy index is negative. If it’s hydrophobic, it’s positive.
We can scan our protein amino acid by amino acid and ask how hydrophobic certain protein segments are.
If it is highly hydrophobic, we see the hump that will suggest that it could be a transmembrane domain.
We can’t determine the exact number of transmembrane domains.
Charged polar amino acids are more often found on the ______ (inside/outside), like in the cytosol or extracellular space.
Outside
Which amino acids are enriched specifically at the polar/nonpolar interface?
Tryptophan and tyrosine. So if you see tryptophan and tyrosine and then a hydrophobic region, that’s a transmembrane domain.
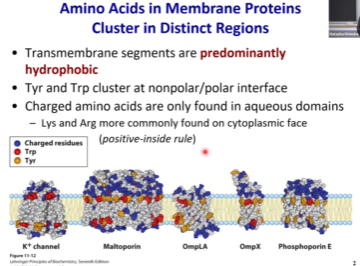
Beta Barrel Transmembrane Structures
FepA - Iron Uptake
OmpLA - phospholipase
Maltoporin - maltose transporter
Transmembrane proteins can come in all structures
Membrane fusion is ______ (favorable/unfavorable). Why? Does the process require activation energy?
Favorable because it increases interactions between different lipids and decreases their interactions with water. The water molecules being removed from polar heads and pushed aside requires some activation energy, which means it’s nice to have proteins to facilitate it.
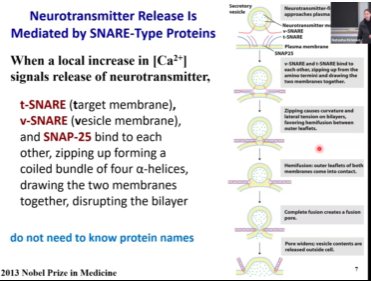
SNAREs
Protein that facilitate membrane fusion belong to the SNARE family
Some of them would be on the target cell plasma membrane Others on the vesicle. Some special SNARES help to form zippers.
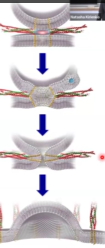
What these SNARE proteins do is they will make a complex, and that complex will start tightening. That will physically push two membranes together and push the water molecules away.
The zipping will cause the membranes to get close to each other and then the formation of hemifusion (when the outer membranes fuse, but not the inner leaflets yet). After that there will be complete fusion where the rest of the membranes fuse as well. After complete fusion, there is formation of a pore that is initially small and then it will widen. And then, the content of the vesicle is added to the content of the cell.
![<p>Realistic SNARE [freebie]</p>](https://knowt-user-attachments.s3.amazonaws.com/4604e664-4e92-4346-a4d3-be93da0f15cb.png)
Realistic SNARE [freebie]
frahbi 😇
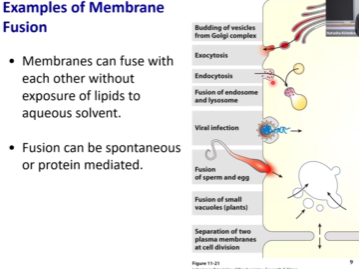
Examples of Membrane-Membrane Fusion
The Golgi is where proteins to be secreted outside of the cells are produced, lots of them are glycosylated. Vesicles need to bud off the Golgi and fuse with the PM and be secreted outside of the cell via exocytosis.
Process of endocytosis allows macrophages to hunt pathogenic bacteria in our bodies to identify and kill them.
In order for cargo inside of endosomes to be degragded, cargo needs to fuse with lysosomes which are rather acidic (pH of 4).
Viruses hijack membrane fusion protein to get inside of the cell.
The process of egg cell and sperm cell merging is also an example of membrane fusion.
Plant vaculoe growing which happens when small vacules fuse with the big ones resultiing in enlargement of plant cells.
These are all very important processes! (well the virus one is bad 🦠 )
Cell Division
The opposite of cell fusion
Membranes break apart to make two new cells out of one cell
Could also be mitochondria dividing to maintain pool of healthy mitochondria in the cell
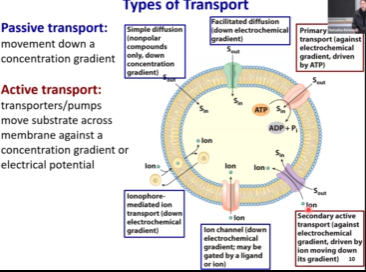
Passive Transport
Refers to transport that does not require any energy
It just happens
When you go down a gradient
When you have a gradient of concentration, systems tend to go to equilibrium. Eventually, their concentration would equilibrize.
Small, hydrophobic molecules can just diffuse through the membrane.
Facilitate transport in a favorable direction
Channels and carriers
Channels can only be passive