Atomic Structure Test - Vocab
1/30
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
31 Terms
Democritus
believed there were small particle called “atoms”
atom = atomos, Greek “indivisible”
believed matter was composed of atoms
had no evidence to support
Dalton
British chemistry teacher
5 theories
“Modern Atomic Theory”
Dalton’s Theory #1
“All matter is composed of even extremely small particles called atoms.”
Modern - atoms have even smaller pieces (quarks, protons, neutrons, electrons)
Dalton’s Theory #2
“Atoms of a given element are identical in size, mass, and other proportions; atoms of different elements differ in size, mass, etc.”
Modern - elements can have atoms of different masses
Dalton’s Theory #3
“Atoms cannot be subdivided, created, or destroyed.”
Modern - they cannot be divided chemically. They can be divided via nuclear power. Ex. - radioactive C-14 becomes N-14 with nuclear power.
Dalton’s Theory #4
“Atoms of different elements combine in simple whole-# ratios to form chemical compounds.”
Modern - yes, but not always the simplest. Non-stoichiometric compounds, like Iron-2 Oxide. Alludes to Law of Definite Proportions.
Dalton’s Theory #5
“In chemical reactions, atoms are combined, separated, or rearranged.
Modern - Conservation of Mass/Matter
J.J. Thomson (1897)
discovered electron using a cathode-ray tube
found electrons are negatively charged because they were attracted to the positive-end of an electromagnet.
most critical development: mass-to-charge ratio of the electron, but couldn’t measure specific values of either.
model: plum pudding model
still supported:
electrons are negatively charged
some neutral particle has to balance it out
now refuted:
arrangement of atom’s structure
Robert Millikan (1913)
oil-drop experiment
discovered the charge of electrons by ionizing () oil droplets and balanced the gravitational pull on the drops.
calculated the charge on the individual drops, which allowed his to find the charge of a single electron.
b/c of this, he could use Thomson’s work to find the mass of an electron.
Ernest Rutherford (1911)
gold foil experiment
sent positively charged alpha-particles towards a piece of gold foil.
alpha particle = type of radiation with a (+2) charge.
conclusions:
atom is mostly empty space, so most a particles passed through the gold atoms
bending is due to the positive charge + mass concentrated in the nucleus
when a particles hit the center, it bounces backwards, if they came to the center, it slightly deflects.
nucleus is made of protons and neutrons.
negatively-charged electrons are located outside the nucleus.
overturned the plum pudding model, showed new atomic structure
Rutherford’s atomic model
atom is mostly empty space
discovery of positively-charged nucleus explained atomic structure + atomic mass concentration
features still supported:
existence of nucleus (p+, n0)
most of atom is empty space
Bohr
linked observations about light, Planck’s quantum theory, Einstein’s explanation on photoelectric effect, and combined the classical thinking of electrons (as tiny specks), he:
proposed a model of the atom in which the electron was able to occupy only certain orbits around the nucleus.
light observations:
white light is a continuous spectrum, ROY G BIV of visible light
excited atoms produce a spectral line/line spectrum, as produced from different amounts of energy.
Planck quantum theory: Energy comes in discrete packets known as quanta. This means that energy can only be transferred in quantized amounts.
Einstein: The emission of electrons from a metal when lights shine on the metal. No electrons were emitted if the light frequency is lower than certain min, no matter how long it shone.
Isotopic notation
AZEn.c.
E= element symbol
Z= atomic #
A= mass #
n.c.= net charge (incl. even if 0)
A = p+n
A-n = p
Protons = Z
p-e = n.c.
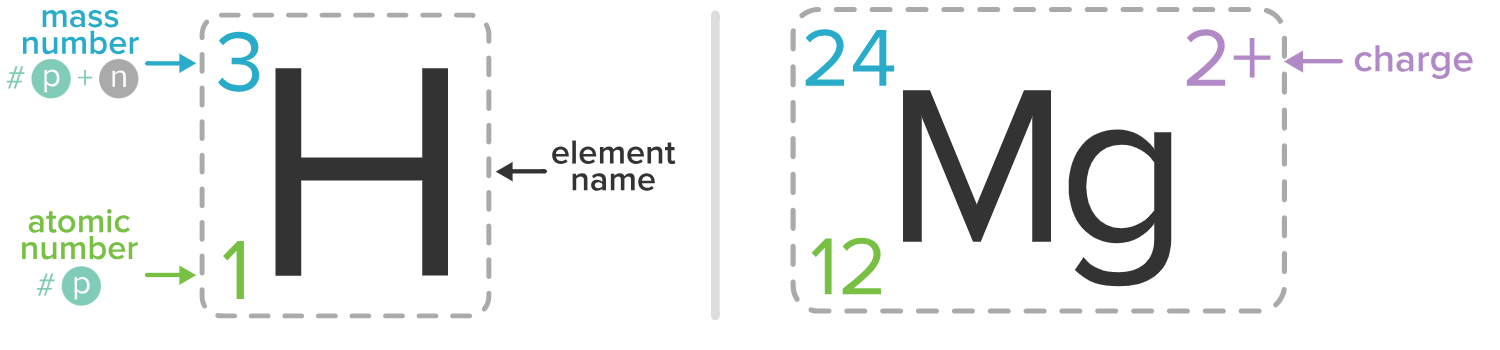
anion
Positive ions (Net charge greater than 0)
cation
Negative ions (Net charge less than 0)
Which part of an atom is changed for an isotope?
Which is changed for an ion?
Iso: number of neutrons (isos have the same # of p+)
Ion: number of electrons
Average Atomic Mass formula
Σ = mi*ai = m1 a1 + m2 a2 …
sum = mass of isotope (in amu) * its decimal abundance
decimal abundance = 90.6% → 0.906
aufbau principle
states that electrons occupy the lowest energy orbitals first before moving to higher energy orbitals.
Electrons fill atomic orbitals in order of increasing energy levels, starting from the lowest (1s) to the highest.
The order of filling is determined by the n + l rule, where n is the principal quantum number and l is the quantum number.
This principle helps explain the electronic configuration of atoms and is fundamental for understanding chemical properties.
Example: For oxygen (O), the electron configuration follows the Aufbau principle: 1s² 2s² 2p⁴.
Pauli exclusion principle
states that no two electrons in an atom can have the same set of four quantum numbers.
Each electron in an atom is described by four quantum numbers: principal (n), angular momentum (l), magnetic (ml), and spin (ms).
As a result, an atomic orbital (the box) can hold a maximum of two electrons, which must have opposite spins.
This principle is crucial for determining the electron configuration of atoms and explains the structure of the periodic table.
Example: In the case of the helium atom (He), which has two electrons, both can occupy the 1s orbital, but they must have opposite spins (one spin-up and one spin-down).
Hund’s rule
states that electrons will occupy degenerate orbitals (orbitals of the same energy) singly and with the same spin before pairing up in the same orbital. (1 ) (1 ) (1 )
This rule minimizes electron-electron repulsion and leads to a more stable arrangement.
When filling orbitals of equal energy, one electron enters each orbital until all are half-filled, then pairing begins.
Hund's rule is essential for predicting the correct electron configurations of elements, particularly those in the transition metals and p-block.
Example: In the case of nitrogen (N), which has three p electrons, the electron configuration is 1s² 2s² 2p³. According to Hund's rule, the three p electrons will occupy the three p orbitals singly (2p_x, 2p_y, 2p_z) before any of them pairs up.
Law of Definite Proportions
(Proust) “constant composition”: A chemical compound always contains the same elements in exactly the same proportion by mass, regardless of the size or source of the sample and its components. The ratio of masses in a compound is always constant. Relates to Dalton #4.
Law of Multiple Proportions
When 2 elements combine to form more than 1 compound, the ratios of the masses of the second element that combine with a fixed mass of the first element are in small whole-#s. Relates to Dalton #4.
Law of Conservation of Mass/Matter
Mass is neither created nor destroyed in a chemical reaction. In any closed system, the total mass of the reactants is equal to the total mass of the products. Relates to Dalton #3, #5.
Democritus vs. Aristotle
Goal of alchemy
n
“principal” or energy level. Relates to the size and energy of an orbital. Starts at 1.
l
“angular momentum” or sublevel. Relates to the shape of an orbital.
l= 0(s), 1(p), 2(d), 3(f), 4(g), 5(h), 6(j), 7(?)
orbitals: s-1, p-3, d-5, f-7, g-9, h-11, j-13
max hold e-: s-2, p-6, d-10, f-14, g-18, h-22, j-26. (rule=*2)
m or ml
“magnetic” or orientation of an orbital in space relative to the other orbitals with the same l. Each sublevel l has 2l+1 orbitals.
s or ms
“spin”. Each orbital holds up to two e-. Filled orbitals have one e- with each spin value: +1/2 or -1/2.
paramagnetism
occurs in materials with unpaired electrons.
diamagnetism
occurs in materials with paired electrons