ocean acidification
1/37
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
38 Terms
explain what is meant by the term ocean acidification
When carbon dioxide is absorbed by seawater, chemical reactions occur that reduce seawater pH, carbonate ion concentration, and saturation states of biologically important calcium carbonate materials. These chemical reactions are termed "ocean acidification".
Calcium carbonate minerals
are the building blocks for the skeletons and shells or many marine organisms. In areas where most life congregates in the ocean, the seawater is supersaturated with respect to calcium carbonate minerals. Meaning there are abundant building blocks for calcifying organisms to build their skeletons and shells.
continued ocean acidification
is causing many parts of the ocean to become undersaturated with these minerals, which is likely to affect the ability of some organisms to produce and maintain their shells.
carbon dioxide and excess heat from fossil fuel combustion
The ocean has absorbed significant amounts of carbon dioxide and excess heat from fossil fuel combustion, causing seas to become warmer, more acidified and stratified with depleted levels of oxygen. Coastal communities around the world are feeling the effects of this change, from oyster die-offs and coral reef bleaching.
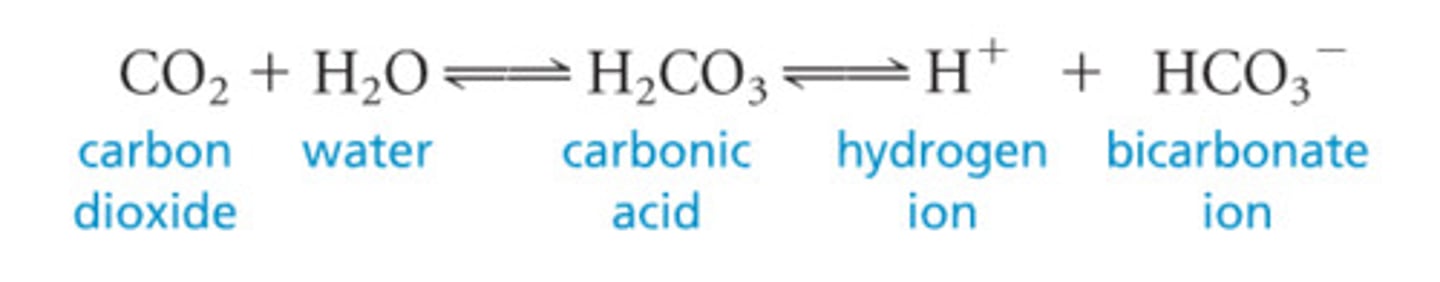
carbon dioxide + water
carbonic acid
carbonic acid dissociates into
bicarbonate and hydrogen ions
Increased carbon dioxide in the air
will dissolve more carbon dioxide into the ocean:
When carbon dioxide enters the ocean,
it dissolves in saltwater. First it forms carbonic acid, then the carbonic acid dissociates producing bicarbonate ions and hydrogen ions.
(partial pressure) of carbon dioxide increases
Therefore if the concentration (partial pressure) of carbon dioxide increases in the air, the forward reaction is favoured and there is a larger yield of hydrogen ions and bicarbonate ions.
Because of human driven increased levels of carbon dioxide
in the atmosphere, there is more carbon dioxide dissolving into the oceans. The oceans average pH is now around 8.1, which is basic, but as the ocean continues to absorb more carbon dioxide, the pH decreases and the ocean becomes more acidic.
Acidity
is a measure (in units of pH) of hydrogen ions in a solution. A higher hydrogen ion concentration will mean the solution is more acidic and its pH would be a smaller number (<7). Therefore higher carbon dioxide present in the air, results in more of it dissolving in the sea water to form hydrogen ions, resulting in the sea water becoming more acidic and therefore a lower pH.
Since the beginning on the Industrial Revolution
the pH of the surface of the oceans waters has fallen by 0.1 pH units. Representing roughly a 30% increase in acidity. Predictions for the future indicate that the oceans will continually absorb carbon dioxide, further increasing ocean acidity. There are estimates that indicate that by the end of this century the surface waters of the ocean could have acidity levels nearly 150% higher.
describe in details four impacts on the ocean of a decrease in pH, using equations where appropriate
Carbon pollution is changing the oceans chemistry, slowing its ability to uptake carbon dioxide, making it more acidic, and harming shellfish and other marine life we depend on
4 impacts
Calcifying species:
Photosynthetic algae and seagrasses:
Coral:
Food chain:
Calcifying species:
As ocean acidification increases, available carbonate ions bond with excess hydrogen ions.
Resulting in fewer carbonate ions available for calcifying organisms to build and maintain their shells, skeletons and other calcium carbonate structures. Calcifying species including oysters, clams, sea urchins, shallow water corals, deep sea corals and calcareous plankton. Existing shells become vulnerable to dissolution. Reducing availability of carbonate ions in ocean water, which provide the building blocks these organisms need to make their shells and skeletons also reduces the chance of survival for their offspring. These species constitute the bottom of the food chain.
Photosynthetic algae and seagrasses:
Photosynthetic algae and seagrasses may benefit from higher carbon dioxide conditions, as they require carbon dioxide to live just like plants on land. It may increase their photosynthetic and growth rates. This may cause a wider variety of biodiversity in the ocean with reference to the photosynthetic plants. It has even been said that seagrasses can mitigate negative ocean acidification effects on calcifying species, by its ability to absorb large quantities of the carbon dioxide, acting as a buffer and increasing the pH of seawater, which could have a positive effect on calcifying species.
Coral:
Increasing ocean acidification has been shown to significantly reduce the ability of reef building corals to produce their skeletons. Research suggests that ocean acidification could severely impact the ability of coral reefs to recover from disturbance, meaning by the end of this century, coral reefs may erode faster than they can be rebuilt. This may compromise the long-term viability of these ecosystems and perhaps impact the estimated one millions species that depend on coral reef habitat. Hinders ability of corals to recover from bleaching events because it reduces the amount of calcium carbonate available that corals needs to grow back to health.
Food chain:
Since life in the ocean is always food for something else, any increase or decrease in the abundance of a species can have a ripple effect on other species. Coral reefs host an abundant and diverse array of marine life, living organisms on which the whole ecosystem depends. They provide habitat and food for a large variety of marine life, including various sponges, oysters, clams, crabs, starfish. Sea urchins and may species of fish. Coral reefs among most biologically diverse and valuable ecosystems on earth. Estimated 25% of all marine life are dependent on coral reefs at some point in their life cycle. Corals may not form calcium carbonate under increased acidity, under severe acidity the coral skeleton can dissolve. Thus effect of increased ocean acidity can have serious consequences for an entire ecosystem. As oceans become more acidic, shelled organisms like oysters, zooplankton and clams have difficulty forming their hard exterior shell, which can lead to decrease in their population. When populations of shelled organisms begin to decline, food for dependent species also begin to decline.
describe two suggestions that have been made to reduce the impact of higher carbon dioxide levels on the oceans.
1. Reduce fossil fuels released into atmosphere
2. Increase ocean carbon sequestration
Reduce fossil fuels released into atmosphere
One way to prevent the rising acidity of the ocean that prevents marine life forming is to reduce peoples carbon footprints. One way to do this through using renewable energies such a solar panels, wind or hydro powers which use little to no carbon to generate energy. Reduced emissions of carbon into the atmosphere will limit the danger to our oceans. More than 150 countries have committed to reduce their fossil fuel emissions in order to prevent increasing effects of climate change.
viability
using renewable energies would be a very viable way to reduce carbon dioxide levels and thus its impact on the oceans as it eliminates using processes that produce the most amount of carbon emissions, however there are limitations based around reliance on the weather (sun and wind) and geographical limitations. There are still challenges to the generation of large quantities of power in renewable energy technology meaning we essentially can't be solely reliant on renewable energy sources to power the entire nation.
2. Increase ocean carbon sequestration
Another suggestion to reduce impact of higher carbon dioxide levels is to remove it from the atmosphere through increase in carbon sequestration. Carbon sequestration is a natural or artificial process by which carbon dioxide is removed from the atmosphere and held in solid or liquid form. This would result in less carbon dioxide dissolving into the ocean, and therefore decrease or slow ocean acidification. The process shows tremendous promise for reducing the human "carbon footprint". Carbon is sequestered in soil by plants through photosynthesis, opens opportunity to store carbon through new land management practises. About 25% of global carbon emissions are captured by plant-rich landscapes such as forests and grasslands. Planting more trees across the world which use photosynthesis and therefore will remove greater amounts of carbon dioxide from the atmosphere and prevent entering of the oceans.
viability -
This is a viable suggestion as it is cost effective, requires minimum amount of resources and human effort, and is a great natural way of solving this issue.
photosynthesis equation
6CO2 + 6H2O ------> C6H12O6 + 6O2
sunlight + chlorophyll
name two other gases that human activity releases into the atmosphere that will also produce acidic solutions if dissolved in water and give the man-made sources of these gases.
The ocean has absorbed 29% of global carbon dioxide emissions since the end of the preindustrial era. In the decade there has been 40 gigatons of emissions of heat-trapping gases each year dumped into the atmosphere, from the burning of fiddling fuels and land-use.
Two other gases that human activity release into the atmosphere is Sulfur dioxide and nitrogen oxide. When these solutions are dissolved in water they form acidic solutions and therefore increase the acidity, and decrease the pH of the ocean.
Sulfur dioxide:
Most of the Sulfur dioxide released into the environment comes from electric utilities, especially those that burn coal. Some other sources of Sulfur dioxide include petroleum refineries, cement manufacturing, paper pulp manufacturing and metal smelting. When Sulfur dioxide combines with water and air, it forms sulfuric acid, which is the main component of acid rain. Acid rain can cause deforestation, acidify waterways to the detriment of aquatic life, corrode building materials and paints
sulfur dioxide + water
SO2 + H2O --> H2SO3
sulfurous acid + water
hydronium ion
HSO4-
Nitrogen oxide:
Gas forms when fuel is burned at high temperatures. Nitrogen oxide pollution is emitted by automobiles, trucks and various other vehicles, as well as industrial sources such as power plants, industrial boilers, cement kilns and turbines. It is a highly reactive gas. When it reacts with water, oxygen and other chemicals in the atmosphere to form acid rain. It's presence in air contributes to the formation and modification of other air pollutants, such as ozone. It is the worst pollutant i the world affecting the oceans because it cases harmful algal blooms, eutrophication and ocean dead zones, makes marine life more vulnerable to disease, reduces biodiversity in shallow estauarine waters, degrades ocean ecosystems and contributes to global warming.
nitrogen dioxide + water
nitric acid + water
outline the Kyoto protocol
Kyoto protocol was adopted in 1997. Owing to a complex ratification process, it entered into force finally in 2005. Currently there are 192 parties to the Kyoto protocol.
United Nations Framework Convention on Climate Change
Kyoto protocol operationalises the United Nations Framework Convention on Climate Change by committing industrialised countries and economies to limit and reduce greenhouse gases emissions in accordance with agreed individual targets. The convention asks those countries to adopt policies on mitigation and report on progress regularly. It only binds developed countries and places a heavier burden on them because it recognises they are largely responsible for the high levels of greenhouse gases emissions.
In Annex B,
Kyoto protocol sets binding emission reduction targets for 27 countries, these targets add up to 5% emission reduction compared to 1990 levels, over the five tear period 2008-12 (first commitment period).
In Qatar 2012
the Doha Amendment was adopted for a second commitment period, starting in 2013 and lasting until 2020. As of October 2020 147 parties deposited their instrument of acceptance, amendment entered into force in December of 2020.
One of the important elements
of Kyoto Protocol was the establishment of flexible market mechanisms, which are based on the trade of emissions permits. Under protocol, countries must meet their targets primarily through national measures. However protocol also offers them an additional means to meet their targets by way of three market-based mechanisms.
It's objective is to achieve
"stabilisation of greenhouse gas concentrations in the atmosphere at a level that would prevent dangerous anthropogenic interference with the climate system. = reduce the emission of greenhouse gases that cause global warming.
6 main greenhouse gases covered by the targets:
- carbon dioxide
- methane
- nitrous oxide
- hydroflurocarbons
- perflurocarbons
- Sulfur hexafluoride