Chemistry IGCSE (Paper 2)
1/295
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
296 Terms
solid
particles are close + touching each other
regular + repeating pattern of particle arrangement
vibrate about fixed positions but don’t move apart
stronger force between particles than in a liquid
not compressible
liquid
particles are close + touching each other - more spaced than solid
irregular particle arrangement
particles move around and slide past each other
forces between particles not as strong as a solid
no fixed shape (takes shape of its container)
not compressible

gas
particles far apart
irregular particle arrangement
particles move freely + collide with each other
very weak forces between particles
no fixed shape or volume
compressible

solid → liquid
= melting
solid heated → particles get energy → vibrate more violently → at certain temp. particles have enough energy to break free from position
liquid → gas/vapour
= evaporating
liquid heated → particles get more energy → move faster, weakening + breaking bonds holding liquid together → at certain temp, particles have enough energy to break bonds
liquid → solid
= freezing
requires significant temp. decrease + occurs at specific temp, different for each substance
gas → liquid
= condensing
gas cooled → particles lose energy → when particles collide, don’t have enough energy to bounce back → group together to form liquid
solid → gas
= sublimation
only happens to a few solids
Potassium manganate + water
Put potassium manganate(VII) at bottom of water beaker
Purple colour slowly spreads out to fill beaker
Particles of potassium manganate(VII) diffuse out among particles of water
Random motion of particles in liquid causes purple colour to eventually be evenly spread out in water
Dilution: If you added more water to final solution of potassium manganate, particles would spread further + solution would be less purple

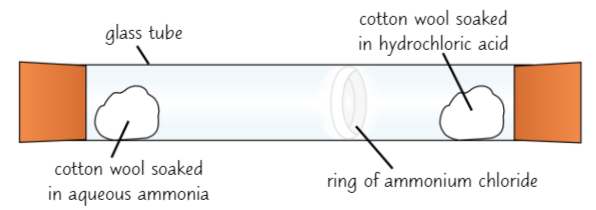
Ammonia + hydrogen chloride
Aqueous ammonia (NH₃) gives off ammonia gas
Hydrochloric acid (HCl) gives off hydrogen chloride gas
Set up experiment as in diagram → white ring of ammonium chloride forms in tube
NH₃ gas diffuses from one end of tube + HCl gas diffuses from other → form ammonium chloride when they meet
Ring doesn’t form exactly in middle - forms nearest end of hydrochloric acid
Because ammonia particles are smaller + lighter so diffuse through air quicker
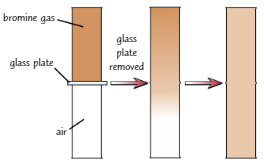
Bromine gas + air
Bromine gas: brown, strong-smelling
Fill half a gas jar full of bromine gas + other half full of air - separate gases with glass plate
Remove glass plate → brown bromine gas diffuses slowly through air
Random motion of particles means that bromine will eventually diffuse right through air
Solute
substance being dissolved
Solvent
liquid that solute dissolves in
Solution
mixture of solute + solvent that doesn’t separate out
Saturated solution
solution where max amount of solute has been dissolved - no more solute will dissolve in solution
Solubility
ability of substance to dissolve solvent
measured in grams of solute per 100g of solvent
solubility of most substances increases with temp
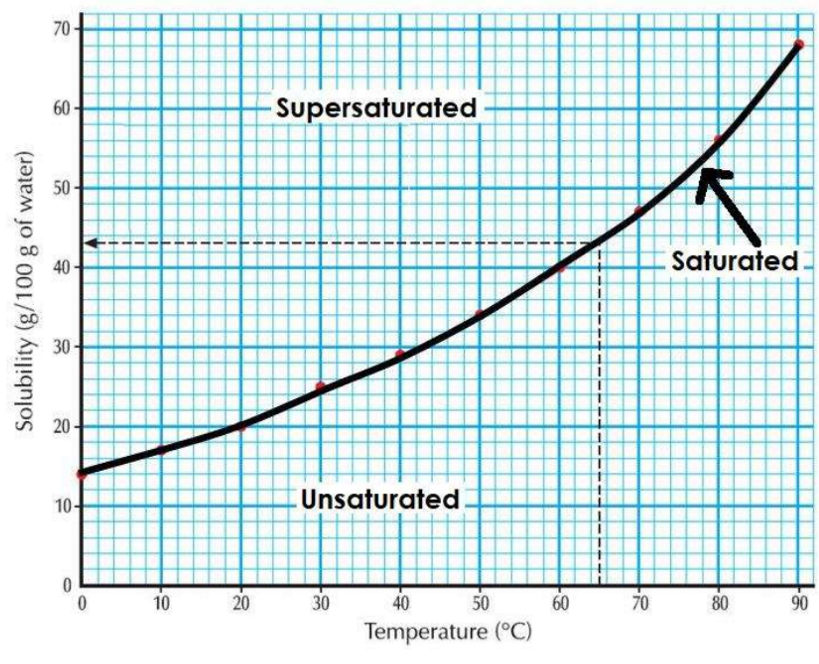
Solubility curve
Can be used to see the effect of temperature on solubility. Shows amount of a solute which dissolves to produce a saturated solution at any given temp.
Solubility curves can be used to predict how much solute will form when we cool a hot solution down.
Investigating how temp affects solubility of ammonium chloride
Make saturated solution by adding excess of ammonium chloride to 10cm³ of water in boiling tube
Stir solution + put boiling tube in water bath at 25ᵒC
After 5 mins, check that all excess solid has sunk to bottom of tube + use thermometer to check solution is at 25ᵒC
Weigh empty evaporating basin, pour some solution into basin
Re-weigh basin + contents, then gently heat using Bunsen burner to remove water - left with pure ammonium chloride
Re-weigh evaporating basin + contents
Repeat steps 1-6 twice but with water bath at diff temps
Use masses to work out solubility at each temp
element
consist of one type of atom only
e.g. oxygen, copper
mixture
material composed of 2+ elements/compounds
physically mixed together
no chemical bond
properties of mixture are mixture of properties of separate parts
e.g. air (mixture of several gases), crude oil (mixture of hydrocarbons, mostly liquids)
compound
made up of atoms of 2+ different elements joined by chemical bonds
properties often totally different from properties of original elements
e.g. carbon dioxide is compound formed from chemical reaction, one C atom reacts with two O atoms to form molecule of carbon dioxide
Pure substance
Made of single element/compound
Has specific melting + boiling point
e.g. pure ice melts at 0ᵒC, pure water boils at 100ᵒCMixture not pure - will melt/boil gradually over range of temperatures
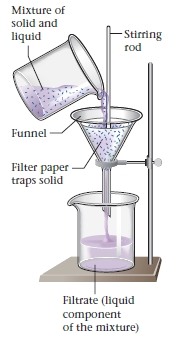
filtration
used to separate insoluble solid from a liquid/solution
Put filter paper in funnel and pour in mixture
Liquid part runs through paper, leaving behind solid residue
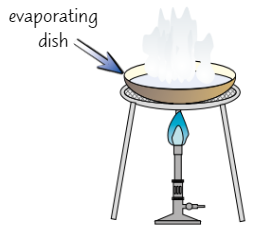
crystallisation
used to separate soluble solid from solution
Pour solution into evaporating dish + gently heat solution
Some water will evaporate, solution becomes more concentratedOnce some water has evaporated/when crystals start to form, remove dish from heat + leave solution to cool
Salt should start to form crystals as it becomes insoluble in cold, high conc. solution
Filter crystals out of solution + leave in warm place to dry
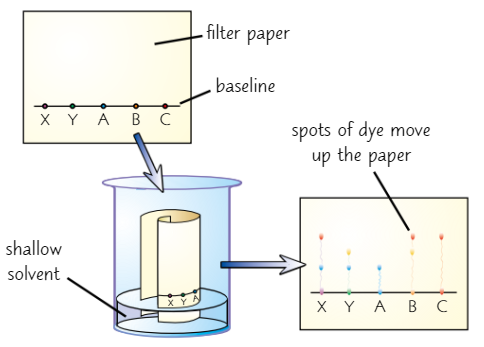
Paper chromatography
used to separate dyes
Draw line near bottom of filter paper (use pencil as pencil marks are insoluble so won’t dissolve in solvent)
Add spots of diff inks to the line at regular intervals
Loosely roll sheet up + put in beaker of solvent e.g. water
Ensure level of solvent is below baseline - don’t want inks to dissolve in solvent
Put lid on container to stop solvent evaporating
Solvent seeps up paper, carrying inks with it
Each dye in inks moves up paper at diff rate + forms spot in diff place
When solvent has nearly reached top of paper, take paper out of beaker + leave to dry
End result is called chromatogram
How chromatography separates mixtures
Different dyes move up paper at different rates
Some stick to paper, others dissolve more readily in solvent + travel quicker
Distance travelled by dyes depends on solvent + paper used
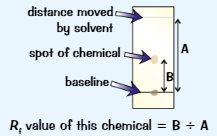
Rf value
Rf = distance travelled by solute/distance travelled by solvent
To find distance travelled by solute, measure from baseline to centre of spot
Chromatography often used to see if certain substance is in mixture
Run a pure sample of substance you think might be in mixture alongside sample of mixture itself
If sample has same Rf values as one of the spots, they’re likely to be same
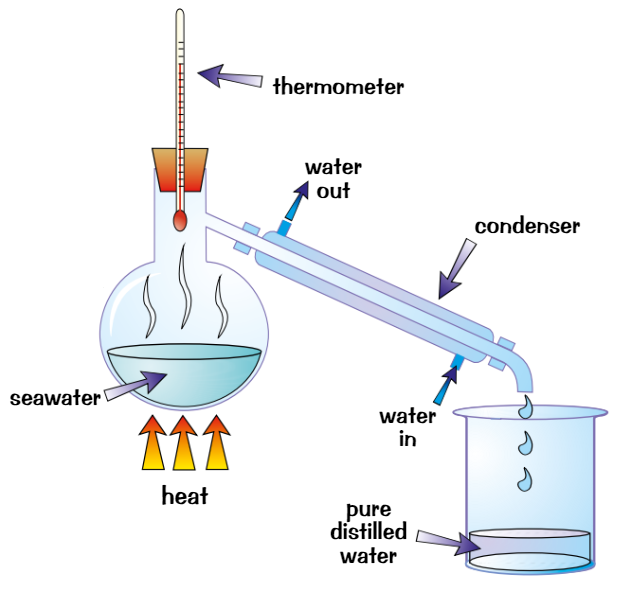
simple distillation
used to separate pure liquid from solution
Heat the solution
Part of solution with lowest BP evaporatesVapour is cooled, condenses + collected
Rest of solution is left behind in flask
Can use simple distillation to get pure water from seawater
Water evaporates, condenses and is collected
Problem: can only be used to separate things with very different BPs
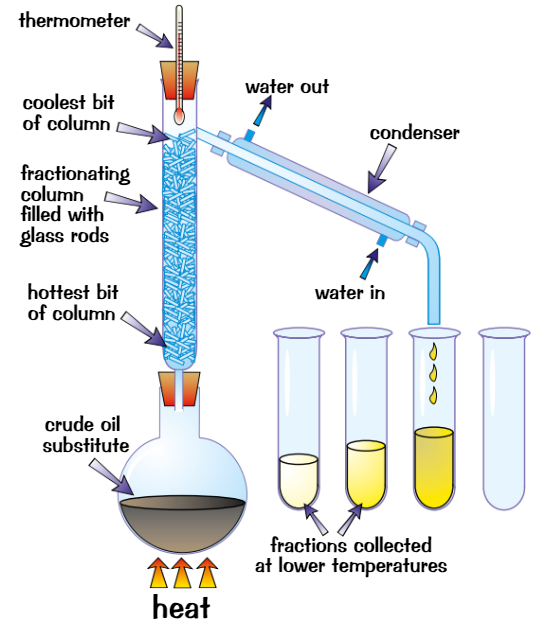
fractional distillation
used to separate mixture of liquids with different boiling points
Put mixture in flask + put fractionating column on top, then heat it
Different liquids have different BPs so evaporate at diff temps
Liquid with lowest BP evaporates first
When temp on thermometer matches BP of liquid, it reaches top of columnLiquids with higher BPs also start to evaporate but column is cooler towards top, so they only get part of the way up before condensing + running back down towards flask
When first liquid has been collected, raise temp until next one reaches the top
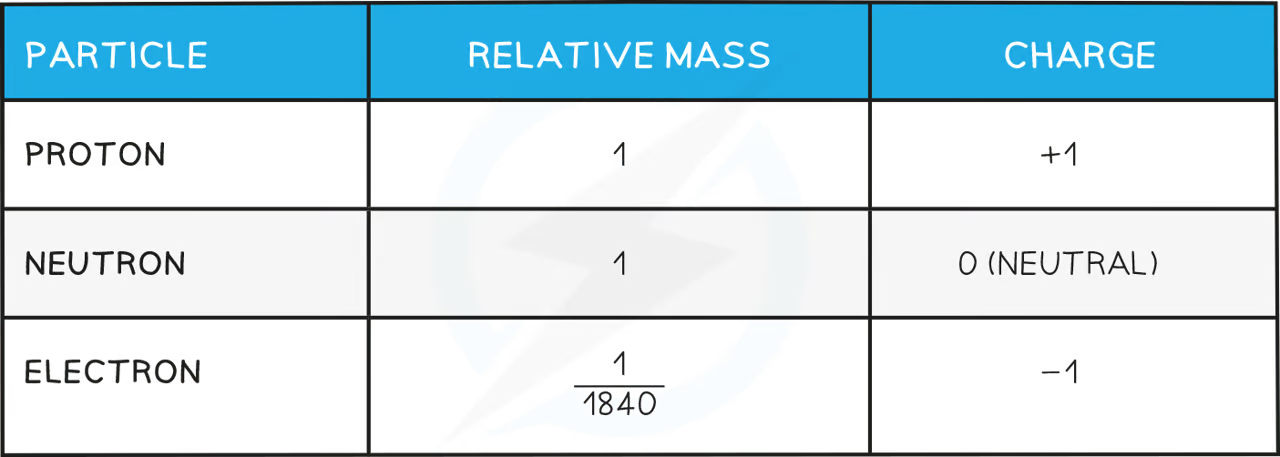
atom
smallest particle of element
consists of electrons surrounding a nucleus that contains protons + neutrons
Sub-atomic particles
molecule
group of 2+ atoms chemically joined together
atomic number
number of protons in nucleus of atom
mass number
sum of number of protons + neutrons in nucleus of atom
isotopes
atoms of same element with same atomic number but different mass number
relative atomic mass
average mass of atom of an element
measured as ratio 1/12 of mass of atom of carbon-12
Calculating relative atomic mass
Multiply % abundance of each isotope by its mass
Add these numbers together
Divide by total abundance (100%)
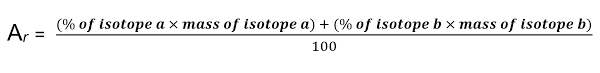
Periodic table
Ordered in order of increasing atomic number
Columns = groups
Rows = periods
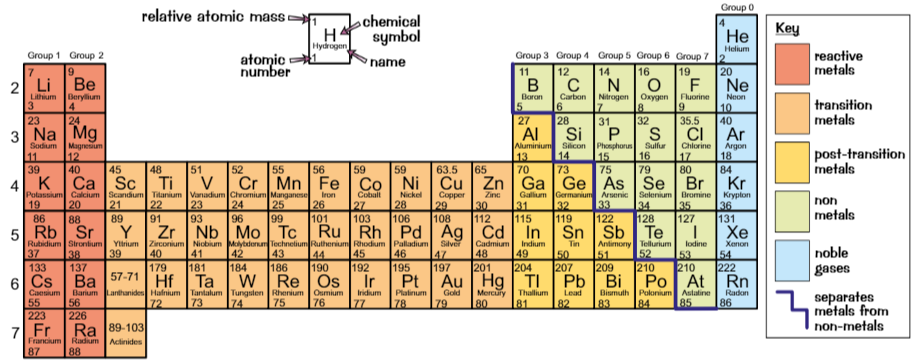
Elements in same group…
have same number of electrons in outer shell
have similar properties
Properties of elements depend on number of electrons
Number of electrons in outer shell is most important
Electron shell rules
Electrons occupy shells
1st shell: 2
2nd shell: 8
3rd shell: 8
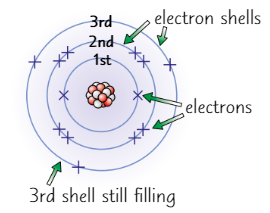
Working out electronic configuration
Period of element = number of shells containing electrons
Group number = number of electrons in outer shell
e.g. Sodium in period 3 so has 3 shells occupied
First two shells must be full (2.8)
In group 1 so has 1 electron in outer shell
So electronic configuration = 2.8.1
Metals
Elements on left of zigzag are metals
Metals conduct electricity because they allow charge to pass through them easily
Metal oxides are basic - they neutralise acids
Metal oxides which dissolve form solutions with pH of 7+
Non-metals
Elements on right of zigzag are non-metals
Non-metals are poor electrical conductors
Non-metal oxides are acidic - neutralise base
Dissolve in water to form solutions with pH less than 7
Group 0
Called noble gases, including helium, neon, argon
Inert - don’t react much
→ takes a lot of energy to add/remove electrons from full outer shell of noble gas atom
Equations show…
Reactants + products of reaction
Can write word equations or chemical (symbol) equations
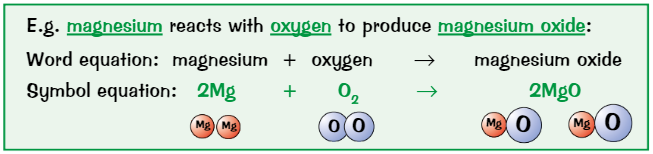
State symbols
(s) - solid
(l) - liquid
(g) - gas
(aq) - aqueous (dissolved in water)
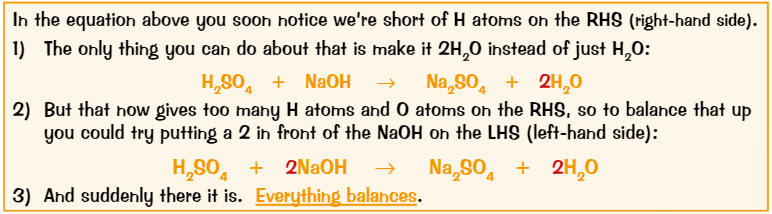
Balancing chemical equations
Put numbers in front of formulas where needed
Find an element that doesn’t balance, write a number to try sort it out
See where it gets you. May create another imbalance, if so, write another number and see where that gets you.
Carry on correcting unbalanced elements until it solves
Relative formula mass
Sum of relative atomic mass of all atoms
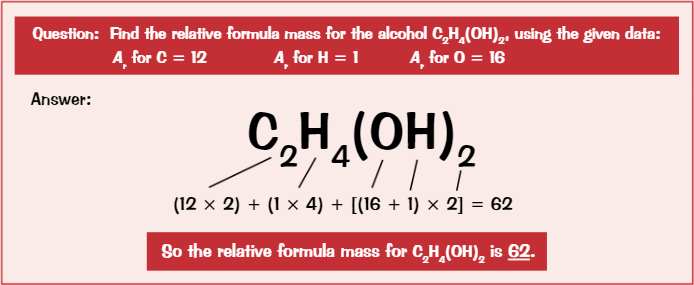
Mole
Unit for amount of substance
One mole of atoms/molecule of a substance has mass in grams equal to relative particle mass for that substance
Molar mass
Mass of one mole, measured in grams
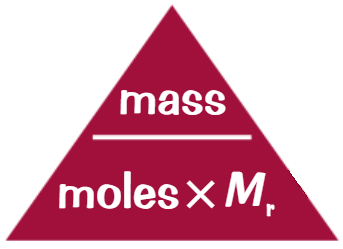
Moles equation
Number of Moles = Mass in g / Mᵣ
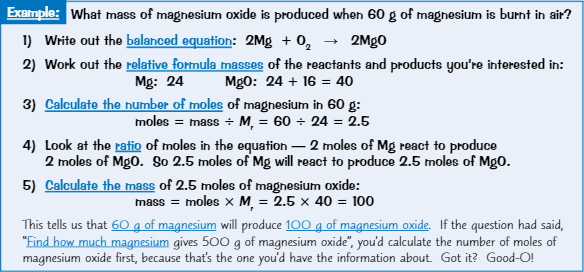
Calculating masses in reactions
Work out balanced equation
Work out Mᵣ of reactant + product you’re interested in
Find number of moles of the substance you know of
Use balanced equation to find how many moles there’ll be of other substance
Use number of moles to calculate mass
Percentage yield
Calculate theoretical yield using balanced equation
Percentage yield = actual yield/theoretical yield x 100
100% yield means you got all the product you expected
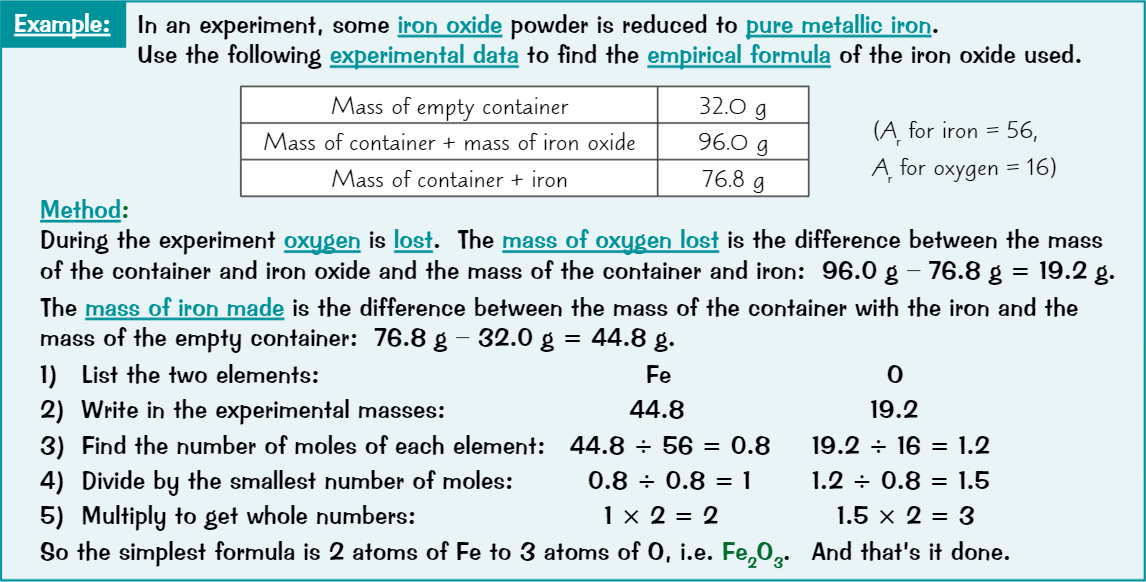
Empirical formula
Smallest whole number ratio of atoms in a compound
Calculating empirical formula
List all elements in compound
Write their experimental masses underneath
Find number of moles of each element
Turn numbers into ratio by dividing by smallest number of moles
Get ratio in its simplest whole number form
Molecular formula
Actual number of atoms of each element in a compound
Calculating molecular formula
Find the mass of empirical formula
Divide molecular mass by formula mass
Multiply empirical formula by number obtained in step 2
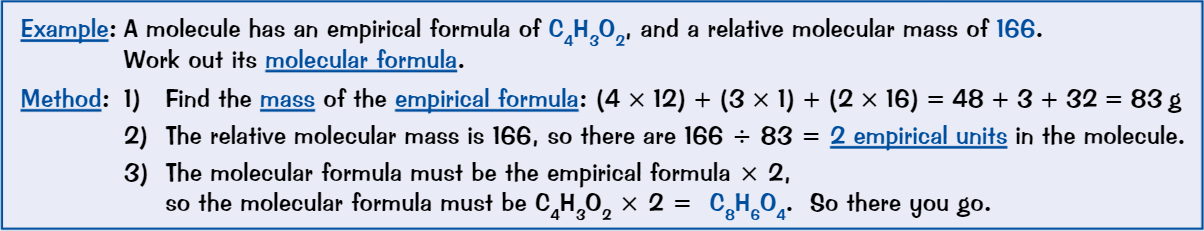
Finding formulae using combustion
Get crucible and heat until red hot (ensures that it’s clean and no traces of oil/water left)
Leave crucible to cool, then weigh it, along with lid
Add some clean magnesium ribbon to crucible
Reweigh crucible, lid and magnesium ribbonHeat crucible containing magnesium
Put lid on crucible to stop bits of solid escape, but leave small gap to allow oxygen to enter crucibleHeat crucible strongly for around 10 mins
Allow crucible to cool and reweigh crucible with lid + contents
Use mass of magnesium oxide and initial mass of magnesium to calculate empirical formula
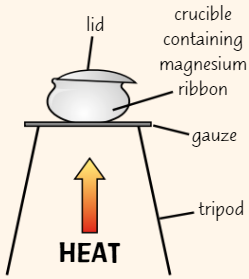
Finding empirical formulae using reduction
Place rubber bung (with hole in middle) into test tube with small hole in end
Weigh them using balanceTake bung out of test tube + spread small amount of copper(II) oxide in middle of tube
Re-insert bung + weigh test tube again
Set up equipment as in diagram
Expel air from test tube by gently turning on gas
After 5 secs, light gas by holding burning splint next to hole in end of tubeUse Bunsen burner to heat copper(II) oxide for 10 mins
Turn off Bunsen burner + leave tube to cool
Once tube has cooled, turn off gas and weigh tube with bung + contents
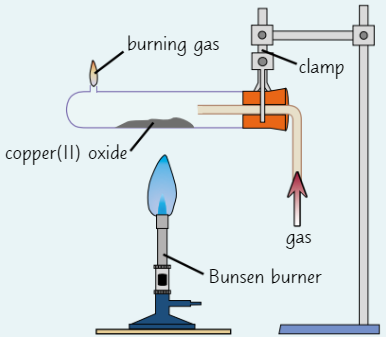
Salts
All solid salts consist of lattice of +ve and -ve ions
In some salts, water molecules are incorporated into lattice
Water in lattice = water of crystallisation
Solid salt containing water of crystallisation is hydrated
If salt doesn’t contain water of crystallisation, it’s anhydrous
Calculating amount of water of crystallisation in a salt
One mole of hydrated salt always has particular number of moles of water of crystallisation - formula shows how many
e.g. hydrated copper sulfate has 5 moles of water for every one mole of salt
So formula is CuSO₄.5H₂O (dot between CuSO₄ and 5H₂O)Many hydrated salts lose water of crystallisation when heated to become anhydrous
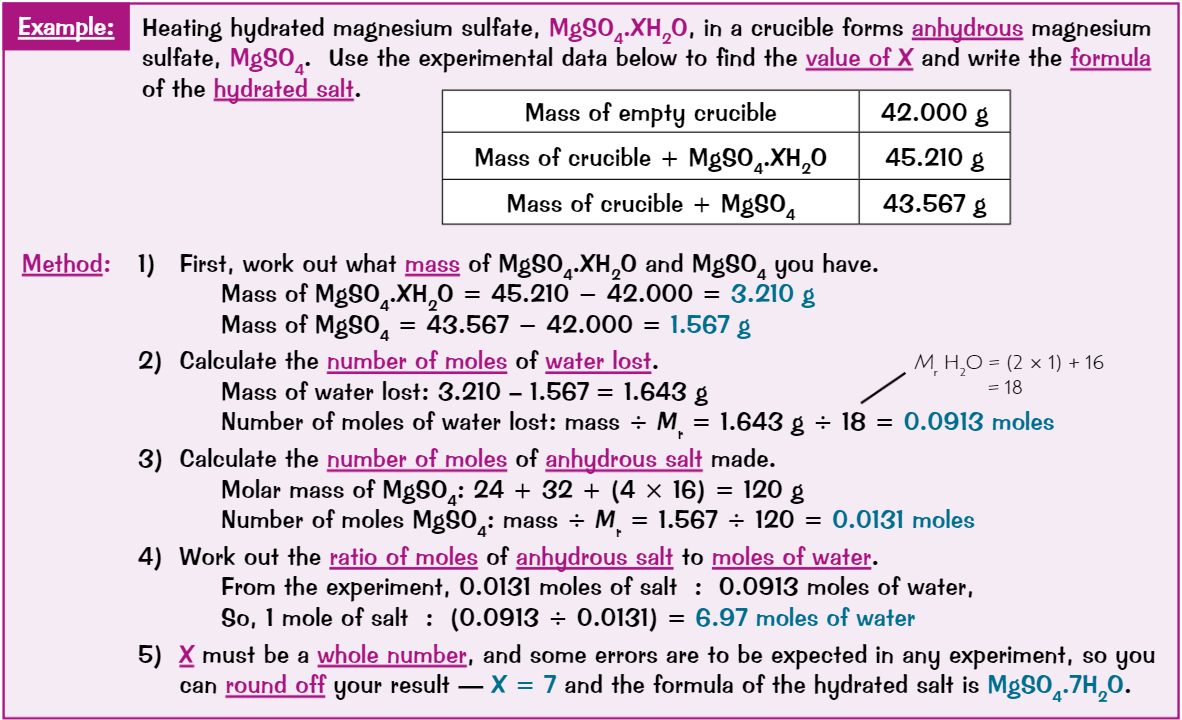
Concentration equation
Concentration (in mol/dm³) = Num of moles (mol) / Vol of solution (dm³)
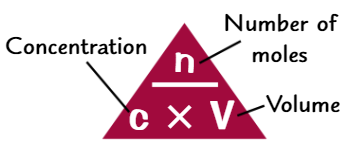
Examples of finding conc. using experimental data
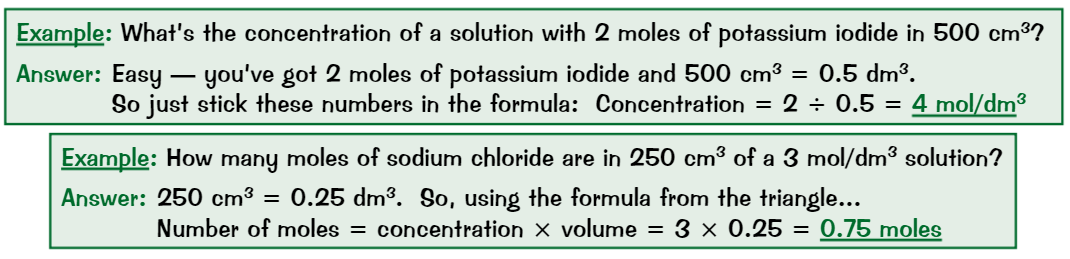
Molar volume
The space that one mole of a gas takes up
One mole of any gas always occupies 24dm³ (=24,000cm³) at room temp + pressure
Volume equation
Volume (dm³) = moles of gas x 24
Calculating volumes in reactions
Calculate moles of substance you know of
Find moles of gas using balanced equation
Convert moles into volume using formula
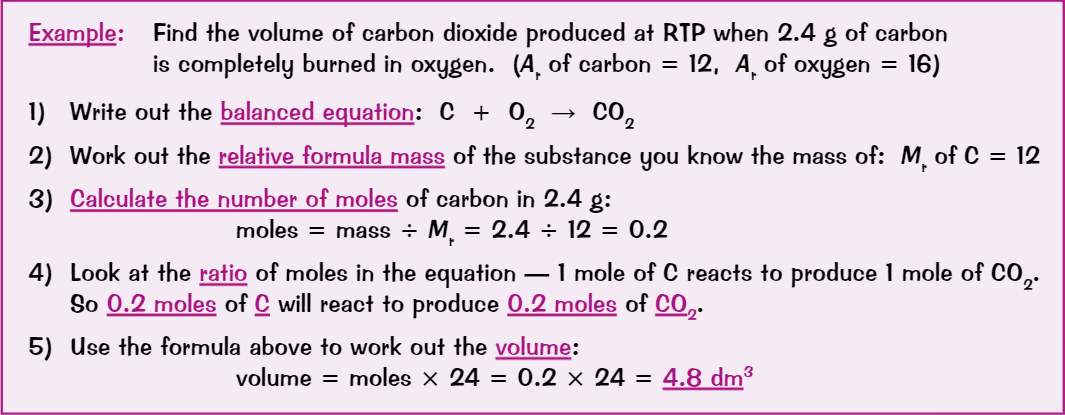
Ions form…
when atoms lose/gain electrons
Negative ions (anions) form when atoms gain electrons
Positive ions (cations) form when atoms lose electrons
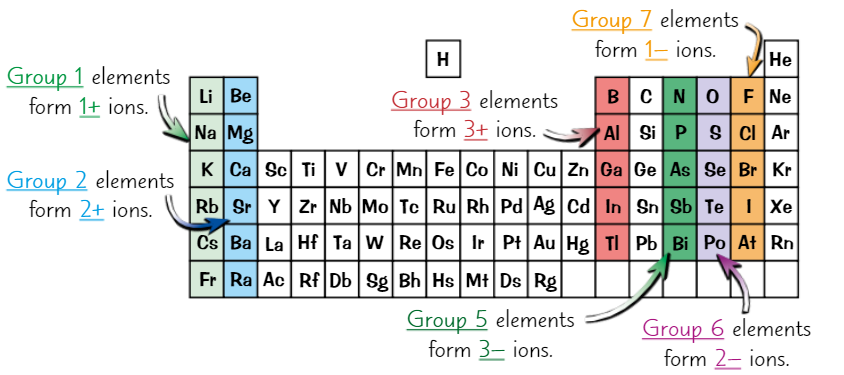
Using group number to predict ions formed
Groups 1, 2, 3 are metals. They lose electrons to form +ve ions.
Groups 5, 6, 7, are non-metals. They gain electrons to form -ve ions.
Elements in same group have same number of electrons in outer shell
So can lose/gain same number of outer electrons
So form ions with same charge
Silver
Ag⁺
Copper
Cu²⁺
Iron(II)
Fe²⁺
Iron(III)
Fe³⁺
Lead
Pb²⁺
Zinc
Zn²⁺
Hydrogen
H⁺
Hydroxide
OH⁻
Ammonium
NH₄⁺
Carbonate
CO₃²⁻
Nitrate
NO₃⁻
Sulfate
SO₄²⁻
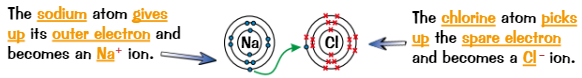
Ionic compounds are produced by…
transfer of electrons
When metal + non-metal react, metal atom loses electrons to form positive ion and non-metal gains these electrons to form negative ion
Formula of ionic compounds
Ionic compounds are made up of positively charged part + negatively charged part
Overall charge of ionic compound = 0
So negative charges must balance positive charges

Ionic dot and cross diagrams
Dots represent electrons from one of the atoms
Crosses represent atoms from the other atom
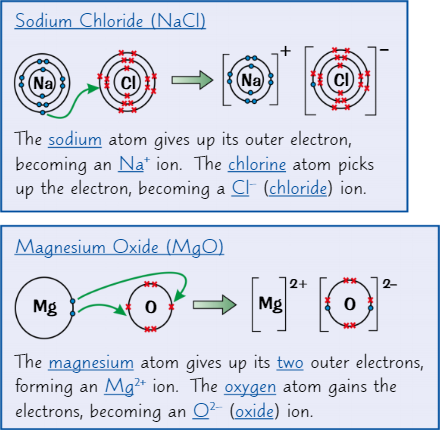
Ionic bond
Electrostatic attraction between oppositely charged ions
Giant ionic lattice
Compounds with ionic bonding have giant ionic structures
Ions held together in closely packed 3D lattice
Electrostatic attraction between oppositely charged ions is very strong
→ a lot of energy needed to overcome strong attraction
→ high melting + boiling points
Ionic compound electrical conductivity
Solid - don’t conduct electricity
Molten/in aqueous solution - conduct electricity
Covalent bond
Atoms make covalent bonds by sharing pairs of electrons with other atoms
Each covalent bond provides 1 extra shared electron for each atom
Covalent bond is…
the strong electrostatic attraction between negatively charged pair of electrons and positively charged nuclei of the atoms involved
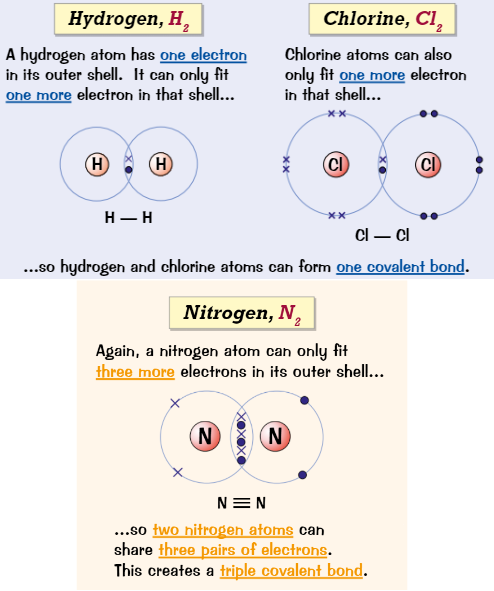
Dot and cross for diatomic molecules
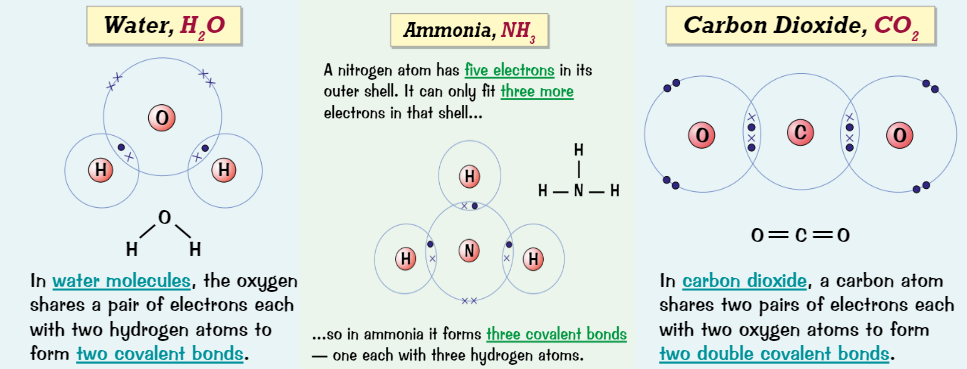
Dot and cross for inorganic molecules
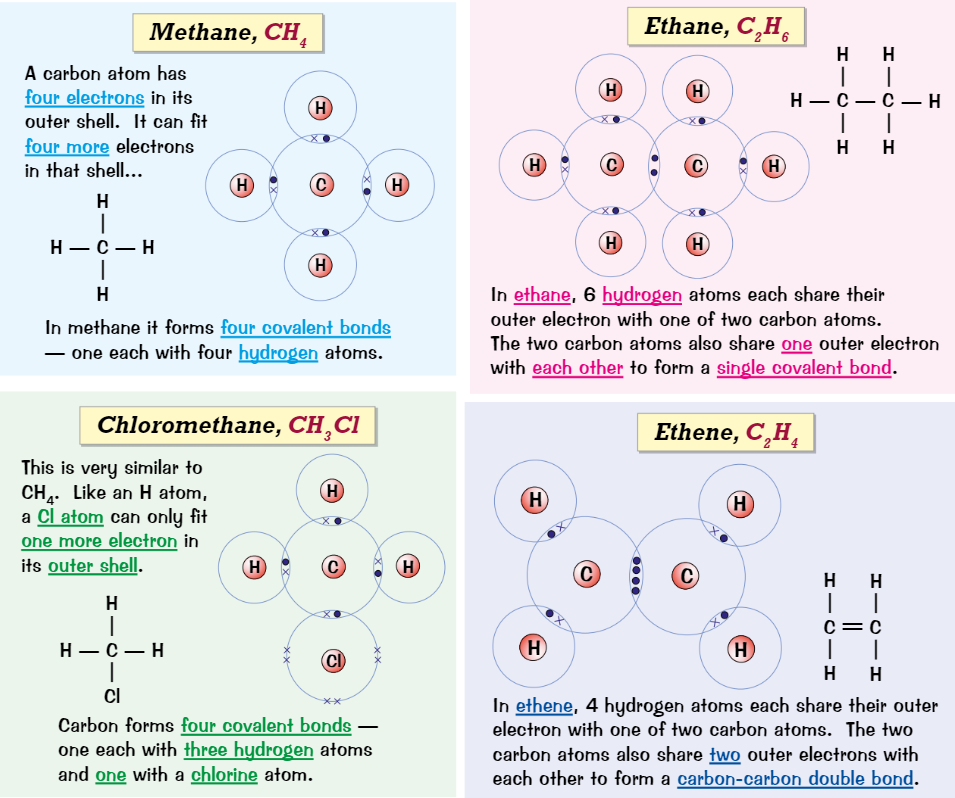
Dot and cross for organic molecules
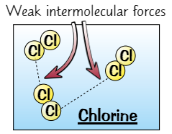
Simple molecular substances
Atoms within molecule are held together by very strong covalent bonds
But forces of attractions between molecules are very weak
→ weak intermolecular forces = very low melting + boiling points, because molecules are easily separated
Are gases/liquids at room temp or solid with low melting + boiling points
Molecules with high relative molecular mass…
have stronger intermolecular forces than smaller molecules
Because there are more points along the larger molecules for intermolecular forces to act between them, so more energy needed to break forces
→ melting + boiling points of simple molecular substances increase as relative molecular mass increases
Giant covalent structures
All atoms bonded to each other by strong covalent bonds
Lots of bonds → takes lots of energy to break them
→ have very high melting + boiling points
Don’t conduct electricity - even when molten (except for graphite)
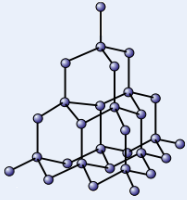
Diamond
Made of network of carbon atoms that each form four covalent bonds
High melting point - strong covalent bonds take lots of energy to break
Very hard - strong covalent bonds hold atoms in rigid lattice structure
Doesn’t conduct electricity - no free electrons/ions
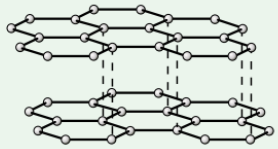
Graphite
Each carbon atom forms three covalent bonds, creating layers of carbon atoms
Soft + slippery - layers are held together weakly by intermolecular forces, so are free to slide over each other
High melting point - covalent bonds in layers need lots of energy to break
Conducts electricity - only 3 out of carbon’s 4 outer electrons are used in bonds, so each C atom has 1 delocalised (free) electron that can move
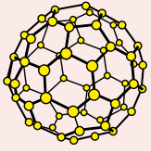
C₆₀ fullerene
Hollow spheres made of 60 carbon atoms
Not a giant covalent structure - made of large covalent molecules
Soft - C₆₀ molecules only held by weak intermolecular forces so can slide over each other
Poor electrical conductor - has 1 delocalised electron but electrons can’t move between molecules
Metallic lattice
Giant structure of positive ions surrounded by sea of delocalised electrons
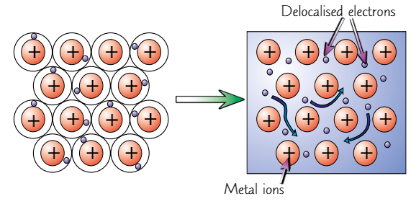
Metallic bonding
Electrostatic attractions between nuclei of positive ions and sea of delocalised electrons
Metal electrical conductivity
Delocalised electrons can move through structure
→ metals can conduct electricity