Vapor-Liquid and Gas-Liquid Separation Processes
1/50
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
51 Terms
More volatile component is…
the one with the lower boiling point
Volatility equation
generally considered CONSTANT
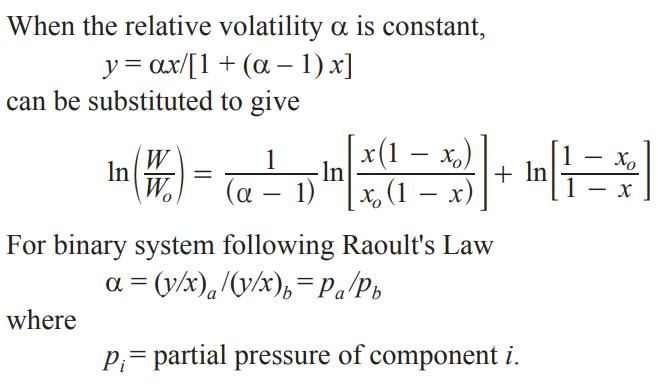
Raoult’s Law
pi=xipi,sat
the partial vapor pressure of a solvent in a solution is equal to the vapor pressure of the pure solvent multiplied by its mole fraction in the solution
Binary flash (equilibrium) distillation (e.g. flash vessel, flash drum, etc.)
→ 6 DOF
energy balance: FHF + Qflash = VHV + LHL
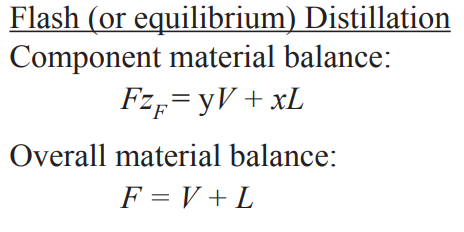
Operating line for binary flash distillation
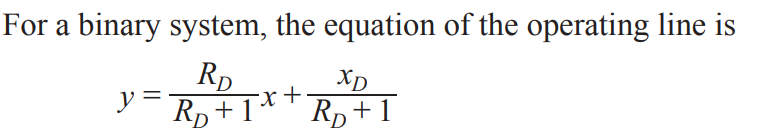
Fraction of feed vaporized (f)
f = V/F
Feed quality (q)
q = L/F
Vapor liquid equilibrium curve (sketch it!!!)
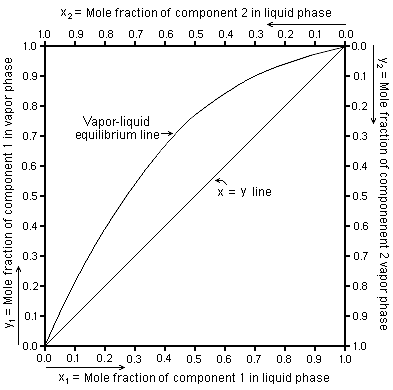
McCabe-Thiele diagram for binary flash distillation (sketch it!!!) → what assumption is required?
relies on the assumption of CONSTANT MOLAR OVERFLOW:
molar heats of vaporization of feed components are EQUAL
every mole condensed → a mole of vapor is condensed
heat & energy effects are negligible (processes adiabatic)
this can be expressed as:
Ln=Ln+1=…=LR
Vn=Vn+1=…=VR
Lm=Lm+1=…=LS
Vm=Vm+1=…=VS
→ w/ assumption, only need 1 operating line
Batch distillation (e.g. still of still pot)
Differential (Simple/Rayleigh) distillation
simplest form of batch distillation → vapor is continuously removed from a liquid, and the composition of the vapor and the remaining liquid (residue) are calculated using the Rayleigh equation
Wo is initial charge
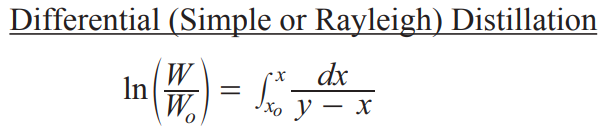
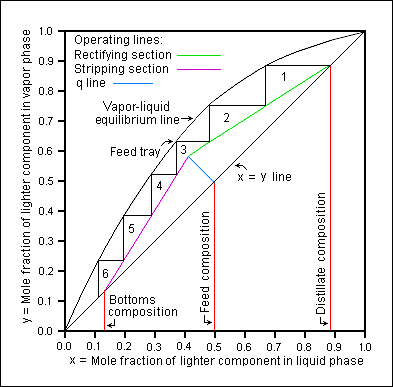
Continuous distillation (e.g. column distillation) → sketch it!!!
→ assumes vapor/liquid leaving each stage is in equilibrium
→ pressure drop between stages is negligible
→ stages operate at different temperatures
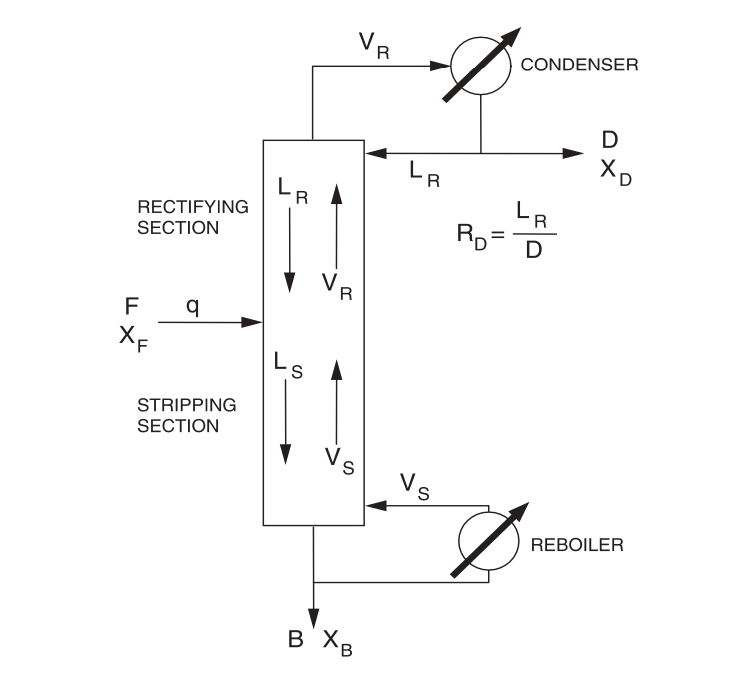
Distillate (D)
Overhead stream → contains a greater concentration of the volatile component
Bottoms (B)
Stream from bottom of column → contains greater concentration of the less volatile component
Partial condensor
operating equation is the same as total condenser
→ ALSO counts as a equilibrium stage at the top of the column (total condenser does not)
Partial reboiler
returns a portion of the bottoms to the column
→ counts as an equilibrium stage
Total reflux
hypothetical condition where all vapor is condensed and all bottoms are returned to column
→ maximum reflux ratio
External reflux ratio (RD)
liquid reflux entering the first stage of distillation (LR) divided by distillate flow rate (D)
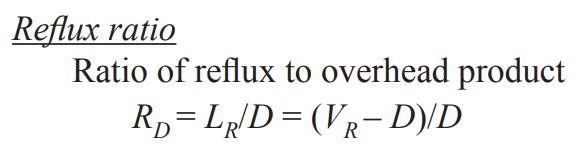
Internal reflux ratio (LR/VR)
liquid reflux entering the first stage of distillation (LR) divided by rectifying flow rate (VR)
= RD/(1+RD)
Minimum reflux ratio (Rmin)
reflux ratio that will result in an infinite number of stages
(graphically, this is where the top operating line touches the equilibrium curve → called pinch point)
Overall (molar) material balance for continuous distillation
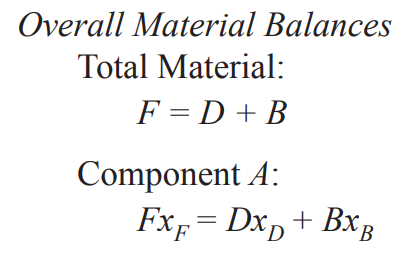
How are trays numbered? (n vs m?)
from to TOP → down
n : rectifying section
m : stripping section
Rectifying (top) section material balance

Stripping section material balance

Operating lines
represents the mass balance relationship between liquid and vapor phases in a column, defined by a linear equation. In the common McCabe-Thiele method, there are two operating lines: one for the rectifying (top) section and one for the stripping (bottom) section
Equilibrium ratio (Kn)
= yn/xn
a function of composition, temperature, and pressure
Where does operating line intersect y = x line?
at y = x = xB
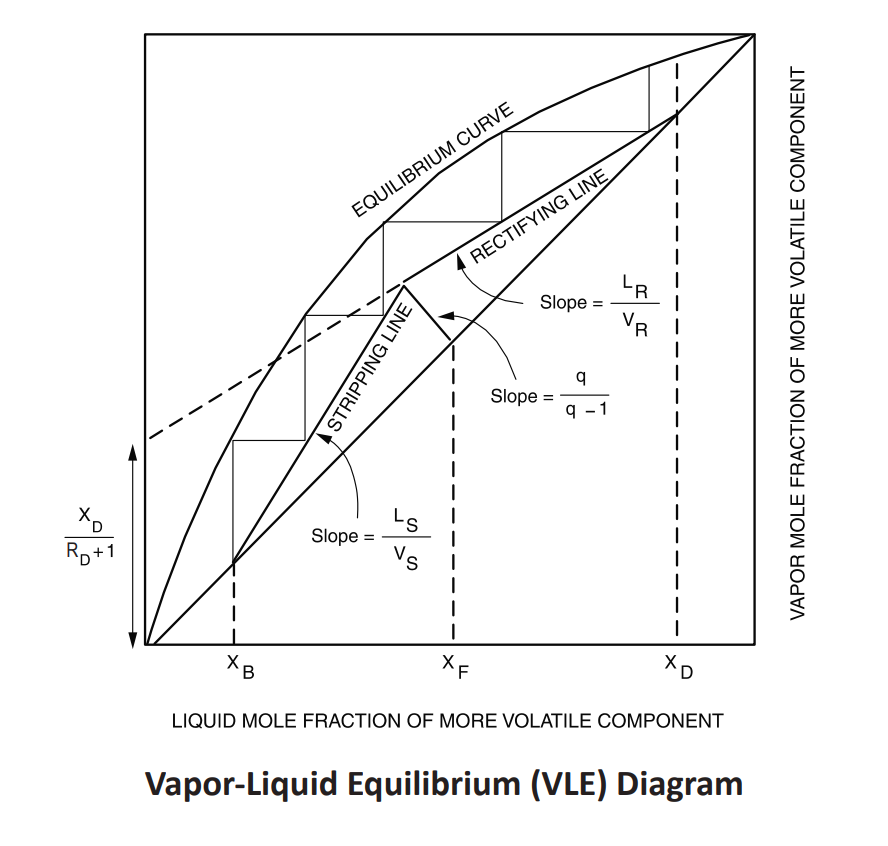
Stepping off stages
draw lines between equilibrium curve and the operating line
rectifying: start at xD and step DOWN
stripping: start at xB and step UP
feed stage location should be placed where operating lines intersect
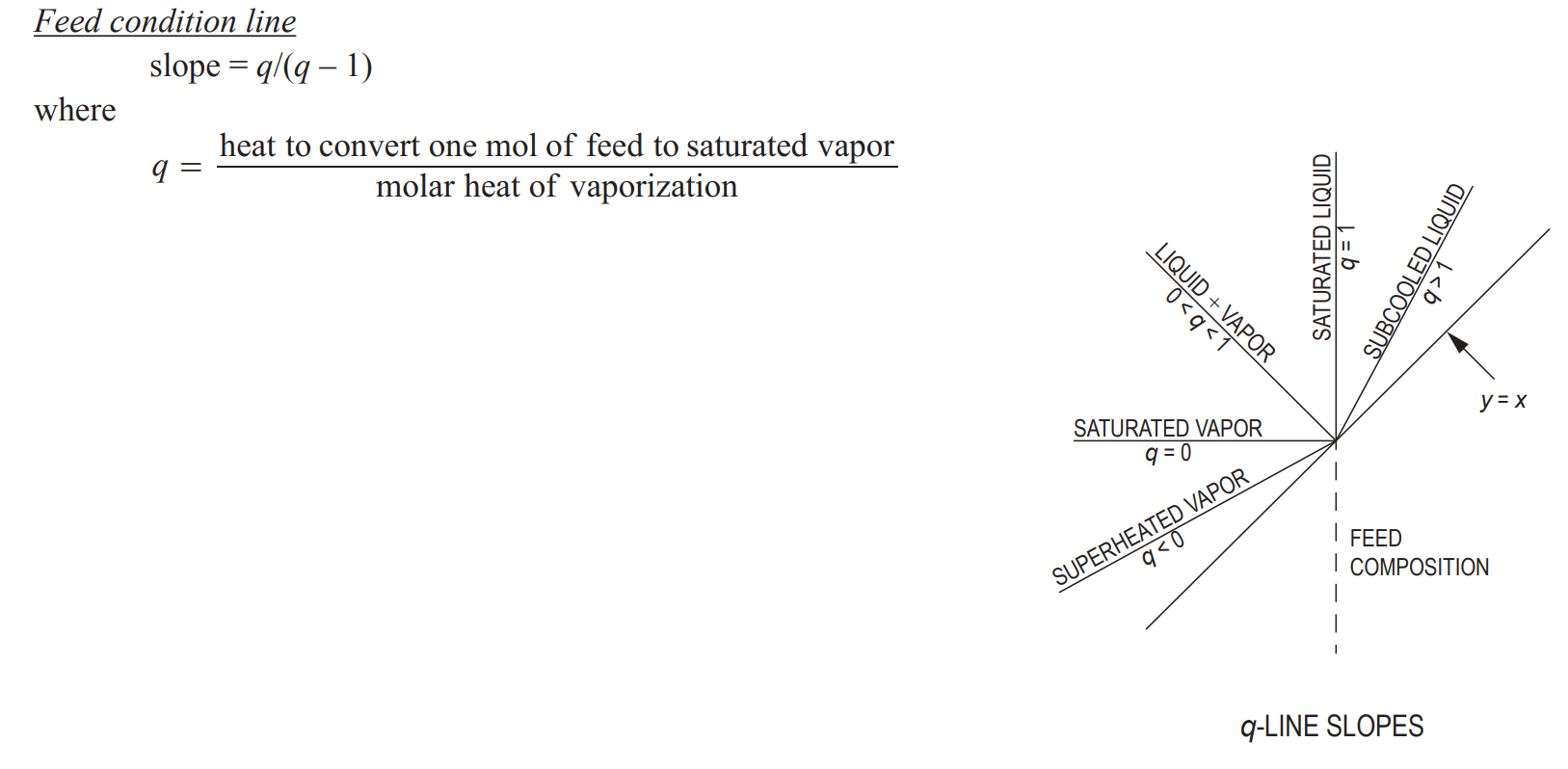
Feed quality (q)
fraction of the feed that remains LIQUID
Feed equation (q)
straight line
q = (hg - h)/hfg
where hfg is latent heat
Fraction of the feed vaporized (f)
complement of the feed quality
Murphree plate efficiency, EME
measure of deviation from actual liquid/vapor composition from ideal (equilibrium on every stage)
→ NOT the same as overall efficiency

Absorption (general)
component in gas stream (solute) is transferred into a stream of a nonvolatile liquid (solvent, separating agent)
→ purified gas is product
Physical absorption
a gas component has greater solubility in the solvent than in its original stream
Chemical (reactive) absorption
the gas component to be removed reacts with and stays with the solvent
Stripping (desorption)
solute from liquid stream is transferred to an insoluble gas stream
→ purified liquid is product
absorption drives mass transfer here too!
Raffinate
purified flow
Extract
dirtied flow
Absorption/stripping in trayed columns: main assumptions
carrier gas is insoluble in the liquid phase
solvent is nonvolatile
the system is isothermal and isobaric
Benefits of trayed columns
can handle wider range of liquid and gas flows
performance and efficiencies predictions are better
easier to install cooling
easier to clean → can be used with liquids that cause fouling or contains solids
Henry’s law
the amount of a gas that dissolves in a liquid is directly proportional to the partial pressure of that gas above the liquid, at a constant temperature
→ valid for LOW solute concentrations and solute partial pressures (< 1 atm)
pA = H’xA
Benefits of packed columns
lower liquid residual (better for flammable or toxic liquids)
better with foaming fluids
less expensive
lower pressure drop
better suited for vacuum operations
What can we assume about pack column flow rates
solvent flow (Ls) and pure gas flow (Gs) are ~constant (NOT total liquid flow, L, or total gas flow, G)
Packing is characterized by what?
surface-to-volume ratio (a)
intrinsic volume drop
which is characterized by packing factor (Cf)
inversely proportional to packing size
HETP?
NEQ?
Height equivalent of a theoretical plate (common measure of efficiency)
Number of equivalent theoretical plates
Porosity (ε)
void fraction of packed bed
ε = (V of voids in bed)/(total V of bed)
Interstitial velocity (vi)
average velocity of fluid through the pores of the column → calculated from superficial velocity, vo
HTU
Height of transfer unit (the smaller, the more efficient the unit) → phase dependent
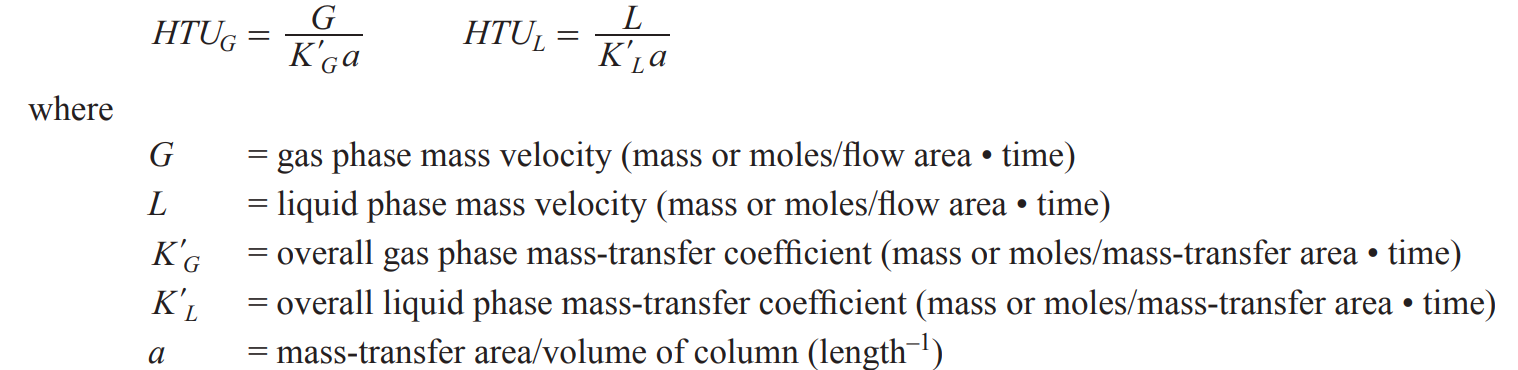
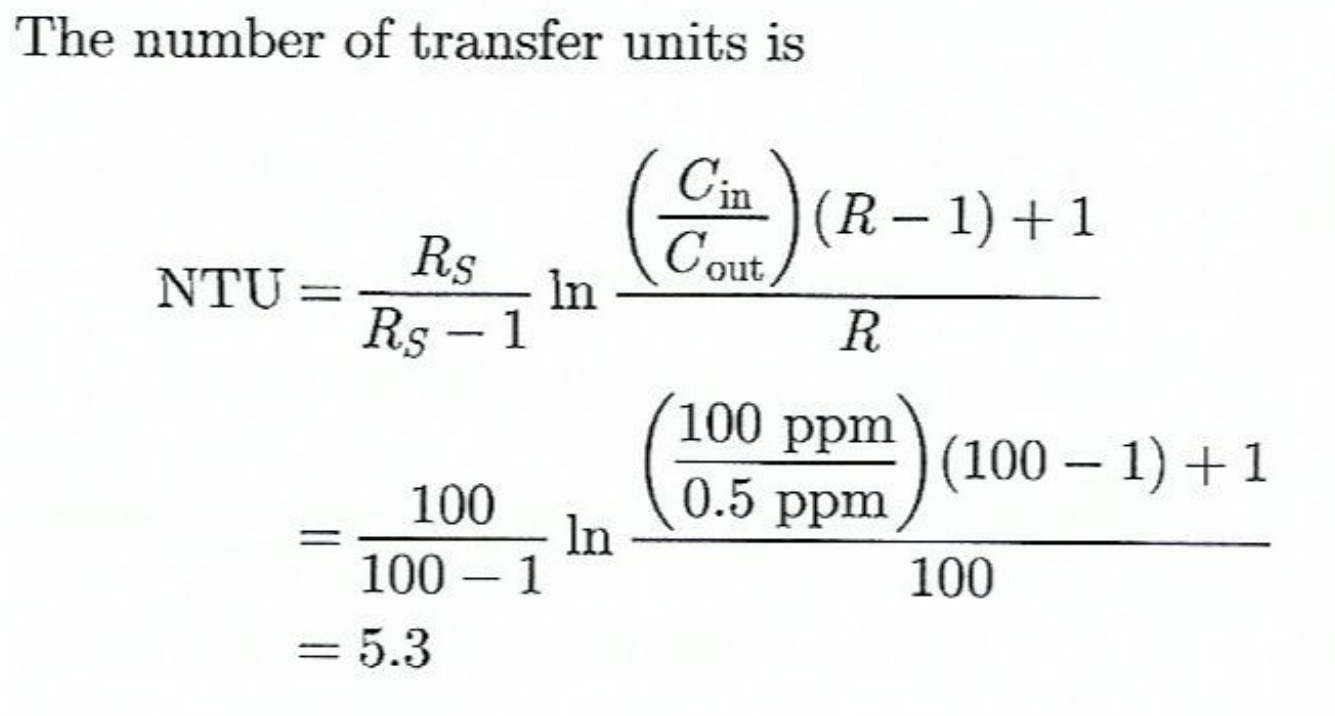
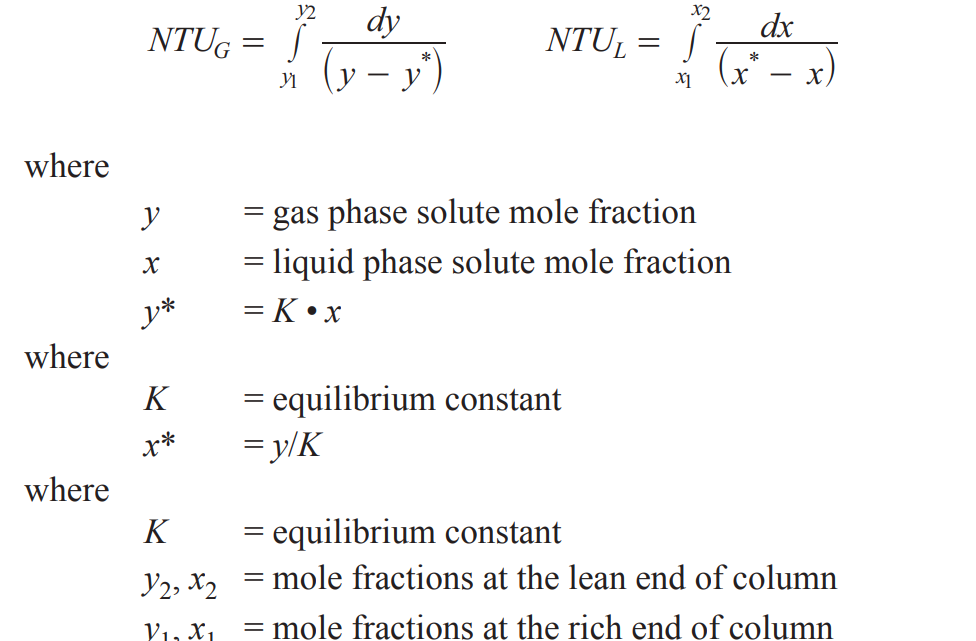
NTU
Number of mass transfer units → phase dependent
Ergun equation use
used to calculate pressure drop through a layer of packing
NTU equation from practice questions