Bio 172 Exam 1 University of Michigan
1/281
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
282 Terms
Properties of Life
1) Order
2) Energy Utilization
3) Regulation or Homeostasis
4) Response to Environment
5) Reproduction
6) Evolution and Adaption
7) Growth and Development
What are peroxisomes?
associated with processes that metabolize hydrogen peroxide and alcohols
break down long chain fatty acids
What is a cell’s nucleus?
where the cell’s chromosomes can be found
large and highly organized
surrounded by double-membrane nuclear envelope
stores and processes information, where DNA is read and converted to RNA by transcription
molecules must be transported in and out of the nucleus
What is the cytoplasm?
everything inside the cell (except nucleus)
cytosol is the fluid portion of cytoplasm
What is the difference between prokaryotic cells and eukaryotic cells?
Prokaryotes lack nuclear membrane and organelles
prokaryotes are usually much smaller and have a fast generation time
What are extracellular structures in prokaryotes?
polysaccharide and protein networks like capsule
for protection
pili and curli
adherence
flagella
for motility
What is light microscopy?
-views of live cells and processes in motion
-in the micrometer range
-can utilize fluorescence to better visualize cells
What is electron microscopy?
pro- resolution is excellent
con- most of the time, what you’re viewing is no longer alive
scanning electron microscopy
specimen coated with thin film, electron beam scans back and forth across sample. Only allows for surface visualization
transmission electron microscopy
cells need to be thinly sectioned to obtain an image
Where can ribosomes be found in a eukaryotic cell?
in the cytoplasm
proteins destined for organelles, cytosol
rough ER
proteins that will stay in the endomembrane system, or exported/secreted from the cell
mitochondria and chloroplasts
How can polar solutes cross the lipid bilayer?
with transport proteins that form channels (pores) or a shuttle system (carriers)
these proteins are specific for their cargo
How does membrane permeability affect molecule movement?
small nonpolar molecules such as O2, CO2, N2 very easily pass through the membrane
small uncharged polar molecules like H2O and glycerol pass through easily
large, uncharged polar molecules have low permeability (glucose, sucrose)
ions have very low permeability (Cl-, K+, Na+)
How can proteins be integrated into a lipid bilayer?
-amphipathically, they will have one nonpolar, hydrophobic layer and one polar, hydrophilic layer
How do membranes vary in composition?
from one species to another
from on organelle to another
phospholipids are different on each side of the bilayer
proteins are different on sides of the bilayer
How do membranes maintain fluidity?
-phospholipids drift within one side of the bilayer
-fluidity and permeability is enhanced by unsaturated fatty acids tails
-cholesterol stabilizes the membrane and is hydrophobic
How are phospholipids organized?
in lipid bilayers
hydrophobic parts will find each other and will NOT be exposed to water
hydrophilic parts will find each other and water
How are biological membranes made?
a fat minus one hydrocarbon chain and plus a polar group
What are triglycerides?
fats, or triacylglycerol (interchangeable names)
glycerol linked to three fatty acids
What is synthesis?
a condensation or dehydration reaction
How do fatty acids link together?
with glycerol
there is an ester linkage between glycerol and a fatty acid
bond to form membranes
What determines the fluidity of fatty acids?
the abundance of double bonds
saturated fats have fewer double bonds and more hydrogen, making them solid at room temperature
unsaturated fats have more double bonds and fewer hydrogens, making them liquid or oil at room temperature
How are polysaccharides used?
fuel storage (usually alpha links)
starch (plants)
glycogen (animals)
structural (usually beta links — VERY stable)
chitin (arthropod exoskeleton)
cellulose (plant cell wall and bacterial extracellular matrix)
peptidoglycan (bacterial cell wall)
Where does chemical energy come from?
from electrons moving from a high energy state to a lower energy state
What are attributes of lower free/potential energy?
-more stable
-less concentrated
-less ordered (more entropy)
-less work capacity
What changes in a proteins’ environment affects hydrogen bonds the most?
pH!, more hydrogens/protons in the environment affect the ability for hydrogens to form these bond
What changes in a proteins’ environment affects ionic bond the most?
salts/ions!! affect ionic bonds the most
What changes in a proteins’ environment affects covalent bonds the most?
temperature! affects covalent bonds the most
hydrogen bonds and ionic bonds are not affected as easily
How are nucleotides or ‘strands’ attached?
with phosphodiester bonds
How is a sugar-phosphate backbone of a nucleic acid able to bond to other s-p groups?
the strand has polarity on each end
5’ phosphate end
3’ hydroxyl end
How do RNA and DNA nucleotides differ?
they differ at the 2’ carbon
RNA nucleotide/ribonucleotide- composed of phosphate group, and ribose sugar (and base), **always has a hydroxyl at the 2’ carbon
DNA nucleotide/deoxyribonucleotide- contains either a ribose or deoxyribose sugar, a base, and a phosphate group, **always has a hydrogen at 2’ carbon
What is the difference between pyrimidines and purines?
pyrimidines- a singular ring
purines- double rings, larger than pyrimidines
What is ribose?
a sugar group of nucleotides
What are the monomers and polymers of carbohydrates?
monosaccharides and polysaccharides
How does a catalyst/enzyme affect a reaction?
does not change amount of energy released (deltaG)
does not change equilibrium constant (Keq)
lowers the activation energy (Ea)
increases the rate of the reaction
the enzyme or catalyst is not changed by the reaction
Explain binding between a substrate and enzyme.
substrates bind through interactions between the enzyme’s R-groups and the substrate, substrate specificity
binding destabilizes chemical bonds in substrate, this lowers the activation energy and reaction will go faster
folded enzymes brings specific amino acids together to form active sites
need to be polar/nonpolar to attach
What are typical properties of enzyme catalysts?
-they are proteins that are substrate-specific
-contains an active site that a substrate will bind to
When do enzymes catalyze reactions?
enzymes only catalyze reactions where deltaG is negative and the reactants have more energy than products
2 or more reactions may need to be coupled so overall delta G is negative and the reactions are spontaneous
Explain ATP and its usage.
ATP is a high free energy molecule and is therefore less stable, more concentrated, more ordered, and has greater work capacity
has high potential energy from the three phosphate groups that are crowded and negatively charged
energy is released when ATP is hydrolyzed (hydrolysis is exergonic and releases energy for work)
What is activation energy?
the amount of energy required for reactants to reach the transition state
this can be overcome by heat or a catalyst
____ in free energy determines chemical reaction characteristics
“change”
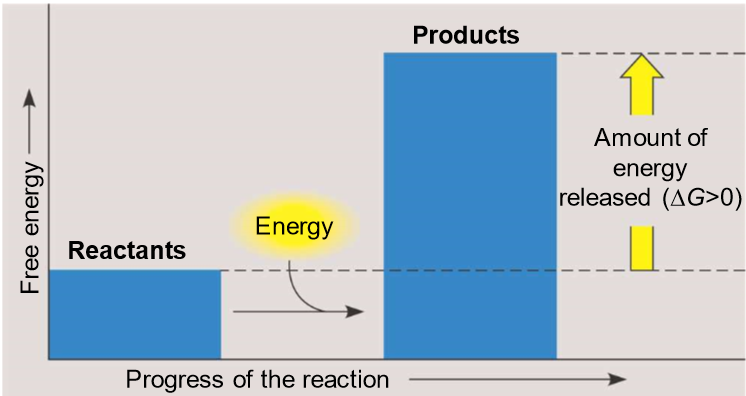
What are energy-releasing reactions?
-exergonic (work)
reactants have MORE energy than products, deltaG is < 0
-exothermic (heat)
these reactions are spontaneous
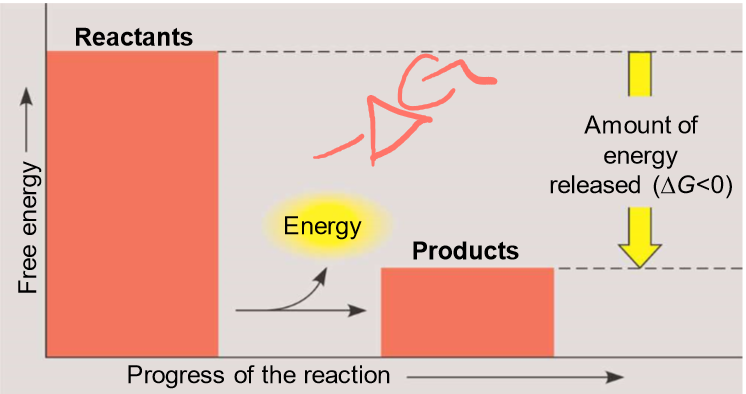
What are energy-consuming reactions?
-endergonic (work)
reactants have less energy than products, delta G is > 0
-endothermic (heat)
these reactions are NON-spontaneous
What is potential or free energy?
energy “stored” in an object
(stored in cells by electron’s chemical bonds)
Where do electrons have the most potential energy?
in the outermost electron shells
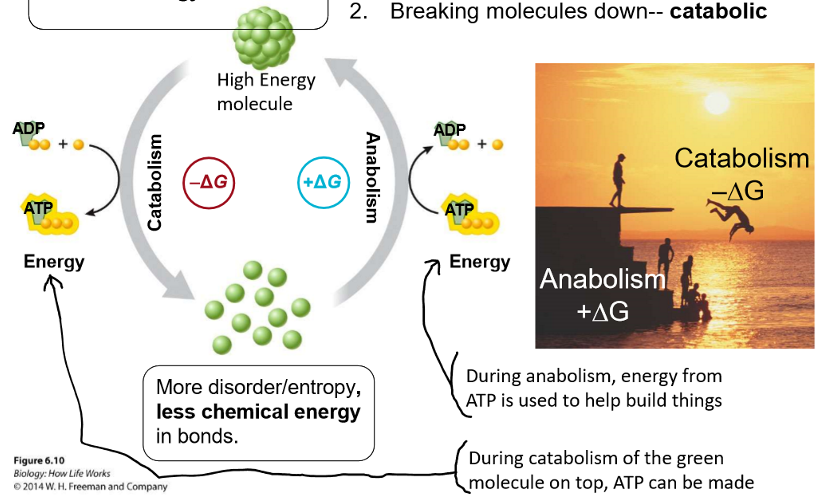
Explain how energy moves through biological systems in a molecular form.
cycle
high energy molecule → catabolism, broken down molecules (-deltaG), ATP can be made → low chemical energy molecule with more disorder/entropy → anabolism, energy/ATP is used to build molecules (+deltaG) → high energy molecule
What is the reaction rate of an enzyme reaction?
Reaction rate = (the amount of product formed (or substrate used))(divided by)/time
What is a basic enzymatic reaction equation?
S + E = ES = E + P
s- substrate
E- enzyme
p- Product
ES is a transition state
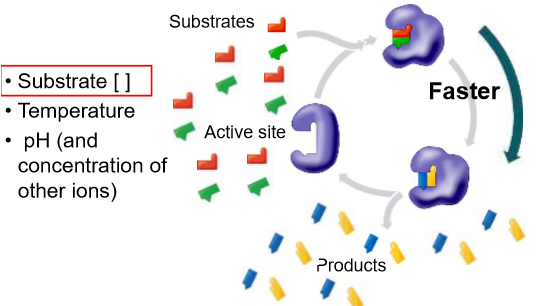
How does concentration affect reaction rates?
To proceed
One or more chemical bonds have to break
Others have to form
Substances must collide in a specific orientation that brings the electrons involved near each other
When the concentration of reactants is high
more collisions should occur
reactions should proceed more quickly
What is v max?
-Enzyme is processing substrate to product as fast as it can (when substrate amount is not limiting)
What happens to reaction rate or velocity as substrate concentration increases?
It will increase and plateau at Vmax
What is Km?
The substrate concentration needed to get ½ the amount of Vmax
The affinity of an enzyme for its substrate
More efficient enzymes have a lower Km
What does having a low Km mean?
The enzyme binds to the substrate tightly and is very efficient at converting it to product
has high affinity
What does having a high Km mean?
The enzyme binds to the substrate loosely and is less efficient at converting it to product
Has lower affinity
How does enzyme concentration affect Vmax?
Less enzyme results in a reduced V Max (fewer products in a given time)
The Km is unchanged
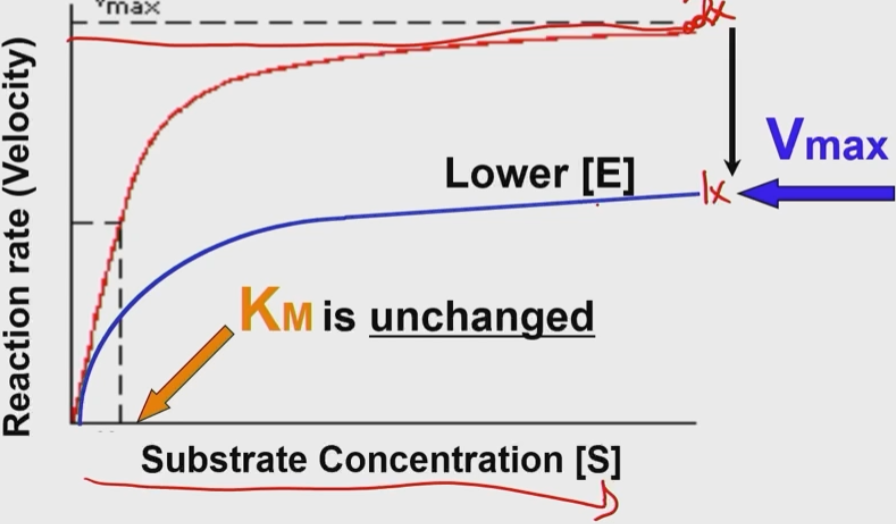
How can we increase v max?
Increase enzyme concentration
Vmax is proportional to enzyme concentration
How does increasing enzyme concentration affect Km?
It doesn’t!
How can enzyme activity be regulated in cells?
Environmental factors such as temperature, pH etc.
competitive inhibitors
noncompetitive inhibitors and activators
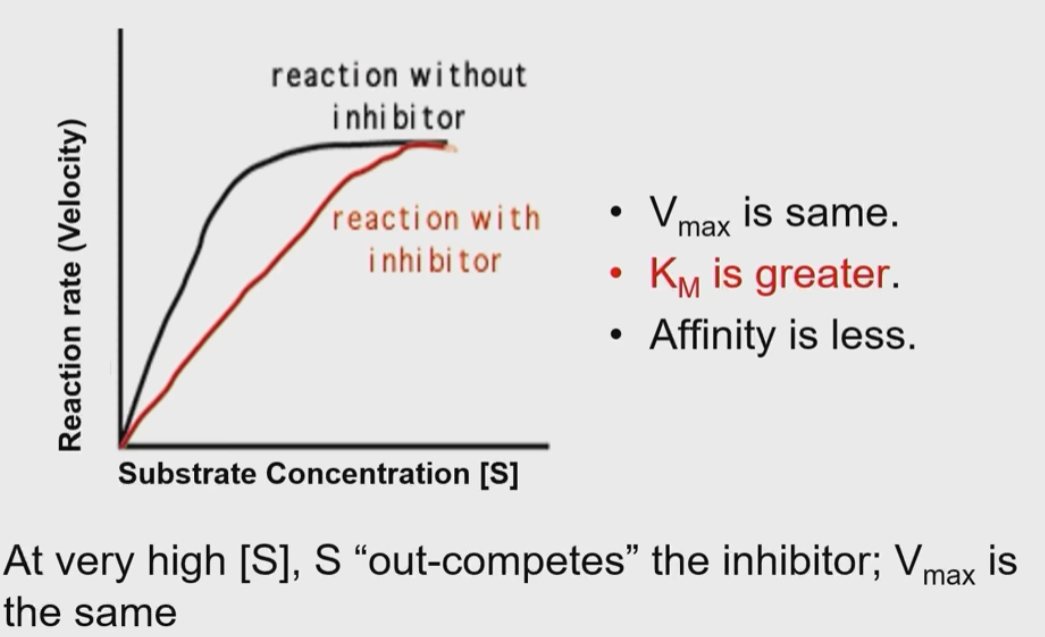
How do competitive inhibitors affect an enzyme’s reaction or function?
They compete with substrate for the active site of enzyme
they often resemble substrate and can fit into the enzyme
at high S, substrates out-compete the inhibitor and effective ness decreases
Km is always changed
What is a real life example of an enzyme competitive inhibitor?
HIV protease - an enzyme that cleaves viral polypeptide into functional proteins (Phe & Pro)
Competitive inhibitor medications can inhibit HIV proteas
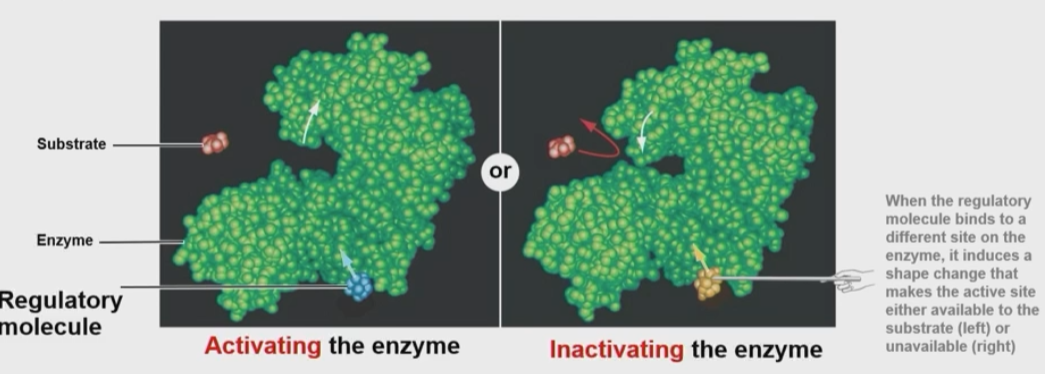
How do non-competitive inhibitors affect an enzyme’s reaction or function?
Can be both positive and negative
Also called allosteric regulation which occurs when a regulatory molecule binds away from the active site
This can activate or inactivate the enzyme by changing its confirmation
It cannot be overcome by excess substrate since the regulator binds away from the active site
Allosteric effectors bind at sites other than the active site, or regulatory site
It alters properties of enzyme function and certain non-competitive inhibitors can affect Km, but most do not
It will always affect Vmax
How can temperature affect the rate of enzyme reaction?
High temperatures cause proteins to unfold
low temperature = low kinetic energy
What does pH affect in the rate of enzyme reaction?
Could affect folding of enzyme
The substrate’s ability to bind to the active site of the enzyme
What factors affect the rate of an enzymatic reaction?
substrate concentration
enzyme concentration
temperature
pH (and concentration of other ions)
Cell
lowest level in hierarchy of biological organization
stores & transmits information
What is specific heat capacity, and what biological molecule has a high SHC?
the energy needed to change the temperature of a substance
water has a very high SHC and needs 1 calorie/gram
What are the aspects of water that facilitate life chemically?
cohesion (H-bonds)
moderation of temperature (high SHC)
insulation by floating ice (reduced density in solid)
solvent for polar compounds
How is does pH affect protons?
acid increases protons
base reduces protons
buffer minimizes changes in protons and hydroxide
Theory of Evolution
All species are related by decent from a common ancestor
Natural Selection
Individuals with heritable traits must survive and reproduce better than individuals with other traits
Tree of Life
A diagram depicting the genealogical relationships of all living organisms on Earth, with a single ancestral species at the base.
Archea
Domain of prokaryotic organisms that are biochemically and genetically distinct from bacteria.
Eukaryotes
membrane-bound nucleus and organelles
unicellular OR multicellular
large
Prokaryotes
Cells that do not contain nuclei, no membrane-bound nucleus/organelles, and are UNICELLULAR
consists of bacteria and archaea
smaller
Are organisms multicellular or unicellular?
Both! Ex. bacteria=unicellular, flowering plant= multicellular
Cell Theory
1) Cells are structural units of life
2) Cells are the functional unit of life
3) All cells are fundamentally similar (ie. structure, metabolic strategies, hereditary info). Specific cell functions vary
4) All cells come from pre-existing cells (via cell growth and division)
Define the attributes of a cell.
enclosed by a plasma membrane that regulates passage of materials in and out of cell
all use DNA for genetic information
What is 1st and most basic level of protein structure?
primary structure
linear sequence of amino acids
held together by covalent bonds (peptide bonds) between the amino acids
What is an atom composed of, and where are these particles?
protons +
neutrons
electrons -
protons and neutrons are in the nucleus, while electrons are found in orbitals surrounding the nucleus
Protons
Have a positive charge
Atomic number
Found in nucleus
Contributes to atomic mass
Neutrons
Has a neutral charge
Found in nucleus
Contributes to atomic mass
Electron
Negative charge
Found in clouds/orbitals
Mass number
the sum of the number of neutrons and protons in an atomic nucleus
Valence
The electrons in the outermost shell (main energy level) of an atom; these are the electrons involved in forming bonds.
Covalent bond
A chemical bond that involves sharing a pair of electrons between atoms to form an orbital
What are the two types of covalent bonds and how do they differ?
nonpolar
electrons are shared equally
polar
electrons are not shared equally, so partial charges exist
What biological molecule is the most electronegative?
oxygen > N > S > C & H
Nonpolar covalent bond
A type of covalent bond in which electrons are shared equally between two atoms of similar electronegativity.
What type of molecules do not easily dissolve in water?
nonpolar covalent molecules
Polar covalent bond
A covalent bond between atoms that differ in electronegativity. The shared electrons are pulled closer to the more electronegative atom, making it slightly negative and the other atom slightly positive.
Hydrogen Bond
A type of weak chemical bond formed when the slightly positive hydrogen atom of a polar covalent bond in one molecule is attracted to the slightly negative atom of a polar covalent bond in another molecule.
Ionic Bond
A chemical bond resulting from the attraction between oppositely charged ions.
Cation
Positive Ion
Anion
Negative Ion
Van der Waals
A slight attraction that develops between the oppositely charged regions of nearby molecules.
Cohesion
Attraction between molecules of the same substance
hydrogen molecules at the surface will hydrogen-bond with the water molecules below; this pulls them downwards
Adhesion
An attraction between molecules of different substances
water molecules at surface adhere to the glass; therefore can resist downward pull of cohesion