Hybridization
0.0(0)
Card Sorting
1/8
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
9 Terms
1
New cards
What is the strongest type of covalent bond?
Sigma bond
2
New cards
How do you calculate the number of electron domains?
Sigma bonds + lone pairs
3
New cards
What is the angle in sp hybridization?
180
4
New cards
What is the angle in sp2 hybridization?
120
5
New cards
What is the angle in sp3 hybridization?
109.5
6
New cards
Single bonds have how many sigma bonds?
1 sigma
7
New cards
Double bonds have how sigma and pi bonds?
1 sigma and 1 pi
8
New cards
Triple bonds have how many sigma and pi bonds?
1 sigma and 2 pi
9
New cards
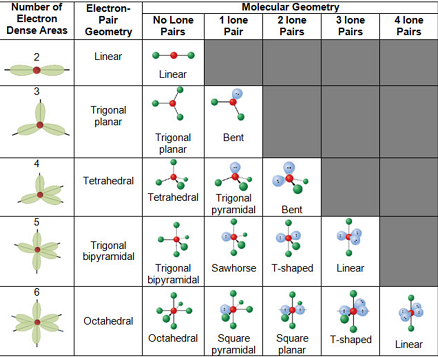
VSEPR Geometry
Know it