Metallic bonding
1/8
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
9 Terms
Why do metals bond metallically?
metallic bonding requires delocalised electrons
The electrons in the outer shells of metal atoms are delocalised
Strong electrostatic attraction between the positive ions and shared negative electrons
These forces of attraction hold atoms together in a regular structure
Metallic bonds are very strong
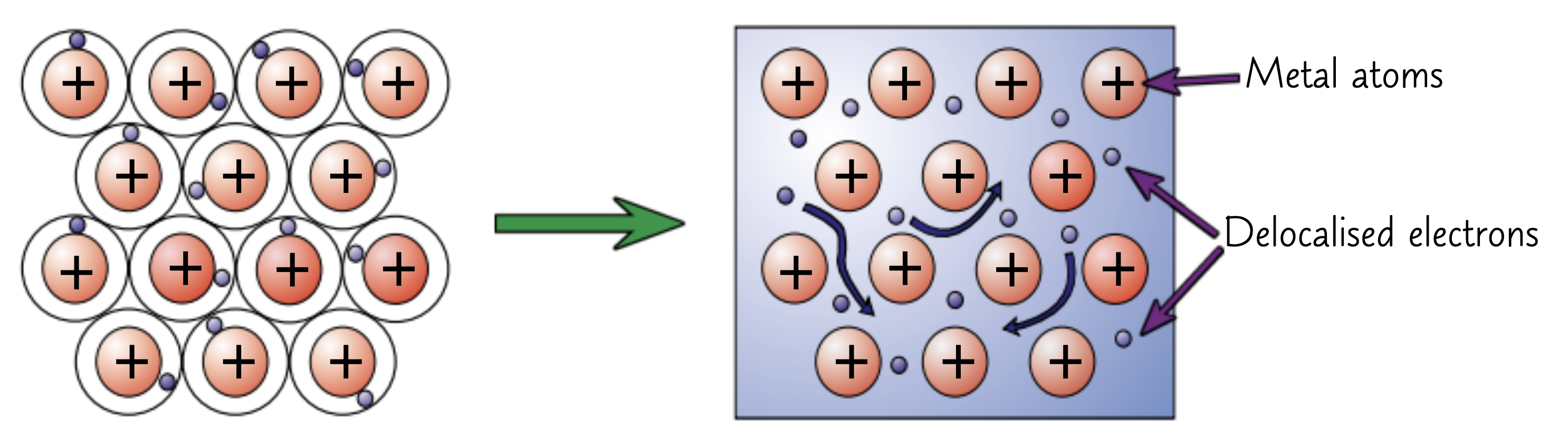
What substances are held together by metallic bonds?
Elements and alloys
Explain why metals are solid at room temperature.
Electrostatic attraction between the metal atoms and delocalised electrons are very strong and require a lot of energy to be broken
Therefore compounds with metallic bonds have high melting and boiling points
What are the properties of metals because of metallic bonds?
Solid
High belting and boiling points
Good conductors of heat and electeicity
Malleable
Why can metals with metallic bond conduct heat and electricity?
The delocalised electrons carry electric charge and thermal energy through the whole structure
Why are most metals malleable because of metallic bonds?
The layers of atoms in a metal can slide over each other
What are some common problems with pure metals?
Not right for specific jobs
too soft
What is an alloy?
a mixture of two or more metals or a metal and another element
Why are allows harder than pure metals?
Different elements have different sized atoms,
when another element is mixed with a pure metal, the new atoms will distort the layers of metal atoms
making it harder for them to slide over each other