ELECTROMAGNETIC RADIATION & QUANTUM PHENOMENA
1/21
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
22 Terms
Photon
A packet of light energy.
Photon energy = hf = hc / 𝛌
Photoelectric effect
When light above a threshold frequency is incident on a metal, electrons absorb photons (one to one relationship) and the electrons gain enough energy to be released from the metal.
Threshold frequency (f0)
Minimum frequency of incident electromagnetic
radiation required to release electrons from the surface of a metal
Work function (ɸ)
Minimum energy required by an electron to escape the surface of the metal
photoelectric effect equation
hf = work function + K.E. max
hf = ɸ + ½ m𝑣2
Stopping potential
The minimum negative voltage required to stop
electrons with the maximum kinetic energy.
Equal to K.E. max / e (= K.E. max in eV)
Ground state
The lowest energy state of an atom.
Excited state
When one or more of its electrons moves to a
higher energy level, the atom is in an excited state.
Excitation
An electron jumps up to a higher energy level of an atom when it absorbs a photon of the right
frequency
Ionisation
When there is sufficient energy for an electron to become excited enough to leave the atom.
Ionisation energy is (photon) energy required for an electron to be freed i.e. to reach n=infinity.
De-excitation
An electron goes down one or more energy levels after a certain amount of time and emits a photon
(with the same energy as the difference between the energy levels).
de Broglie wavelength
Wavelike behaviour of a matter particle characterised by a wavelength, λ = h/(mv)
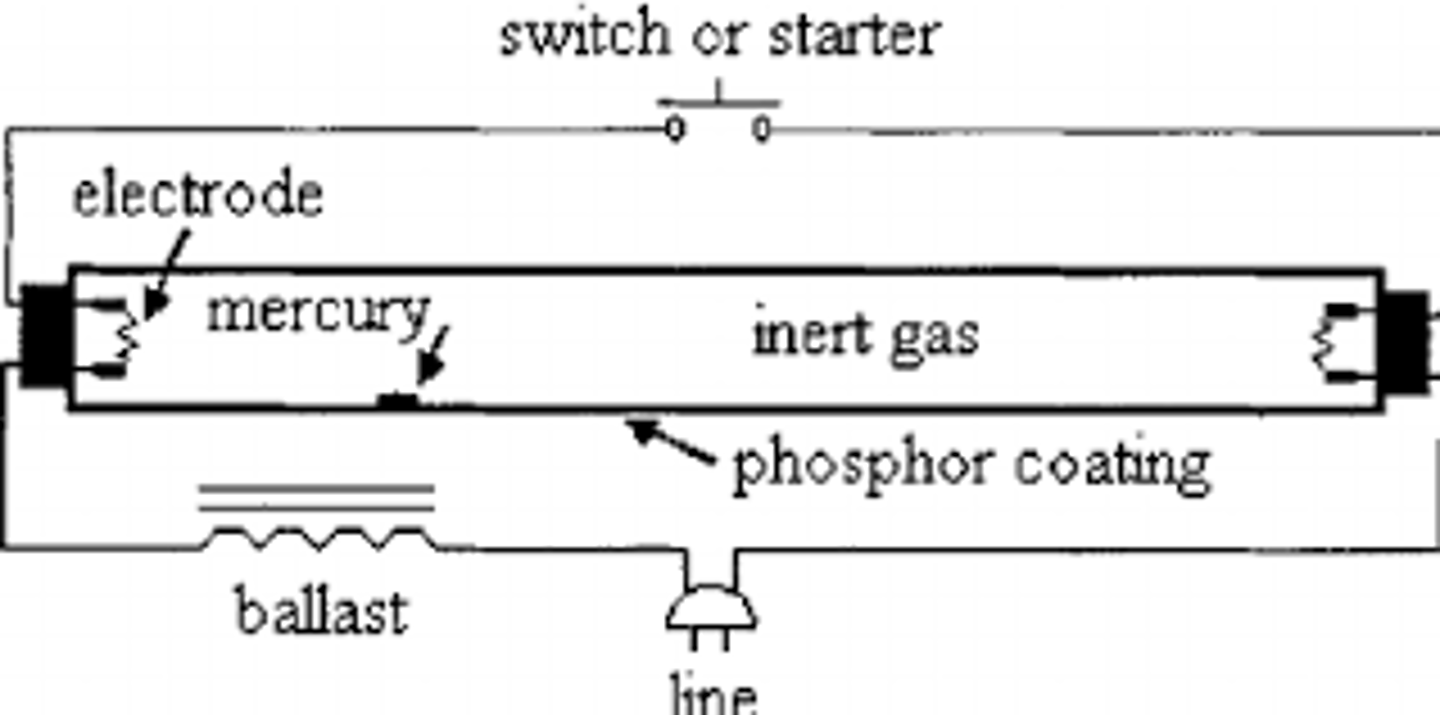
Fluorescent tube
1. High voltage applied across tube, e⁻ flow from cathode to anode, producing e⁻ beam
2. Beam e⁻ collide w/ e⁻ in Hg vapour transferring Ek
3. e⁻ in Hg are excited & move to higher energy level
4. High level = unstable so e⁻ de-excite emitting photons in UV range
5. UV photons collide w/ e⁻ in phosphor coat & get excited to higher energy level
6. Phosphor e⁻ de-excite, emit photons in visible light range
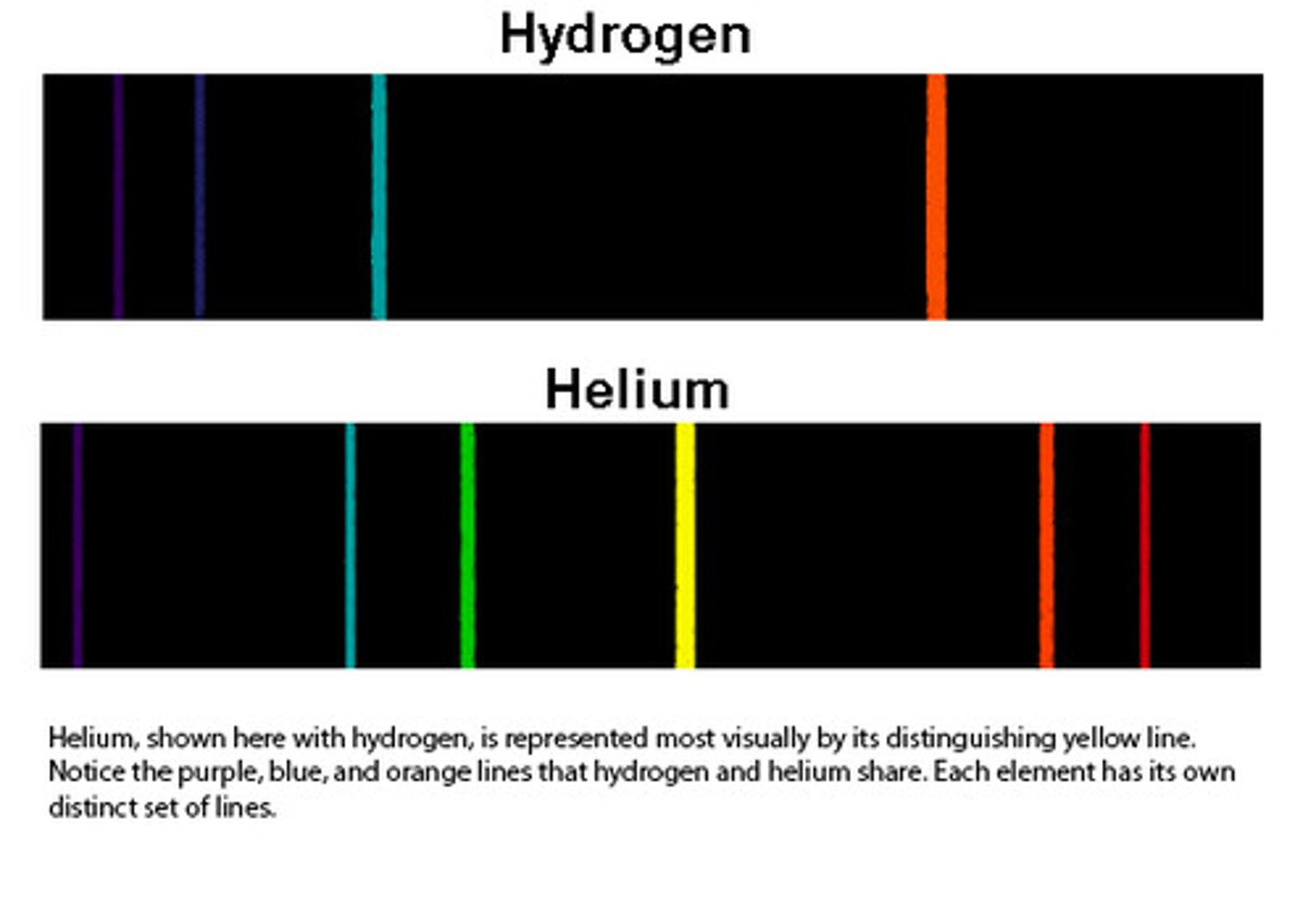
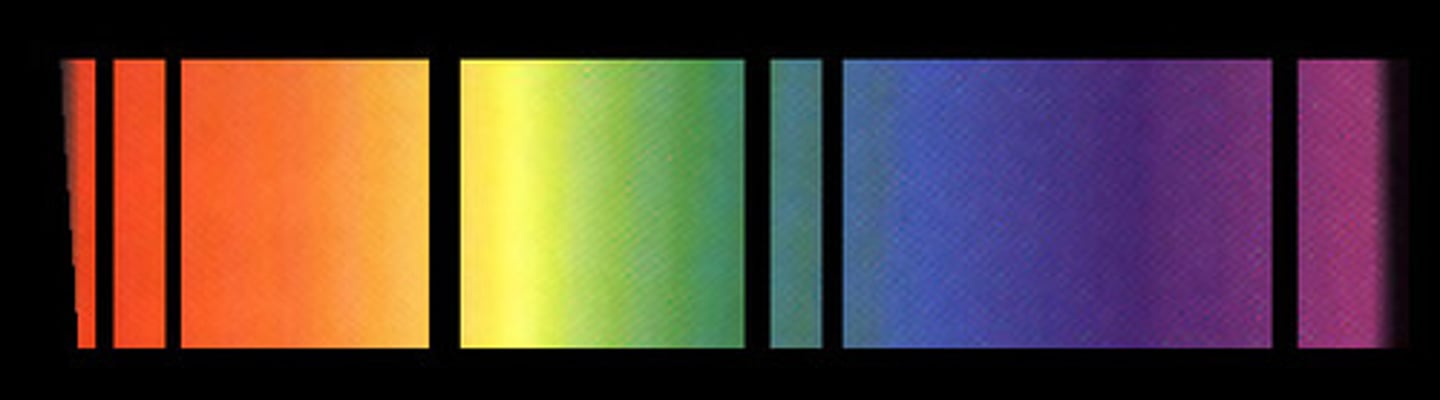
Line spectra
When excited atoms emit light of certain wavelengths which corresponded to different colours
- provides evidence that electrons in atoms can only transition between discrete energy levels
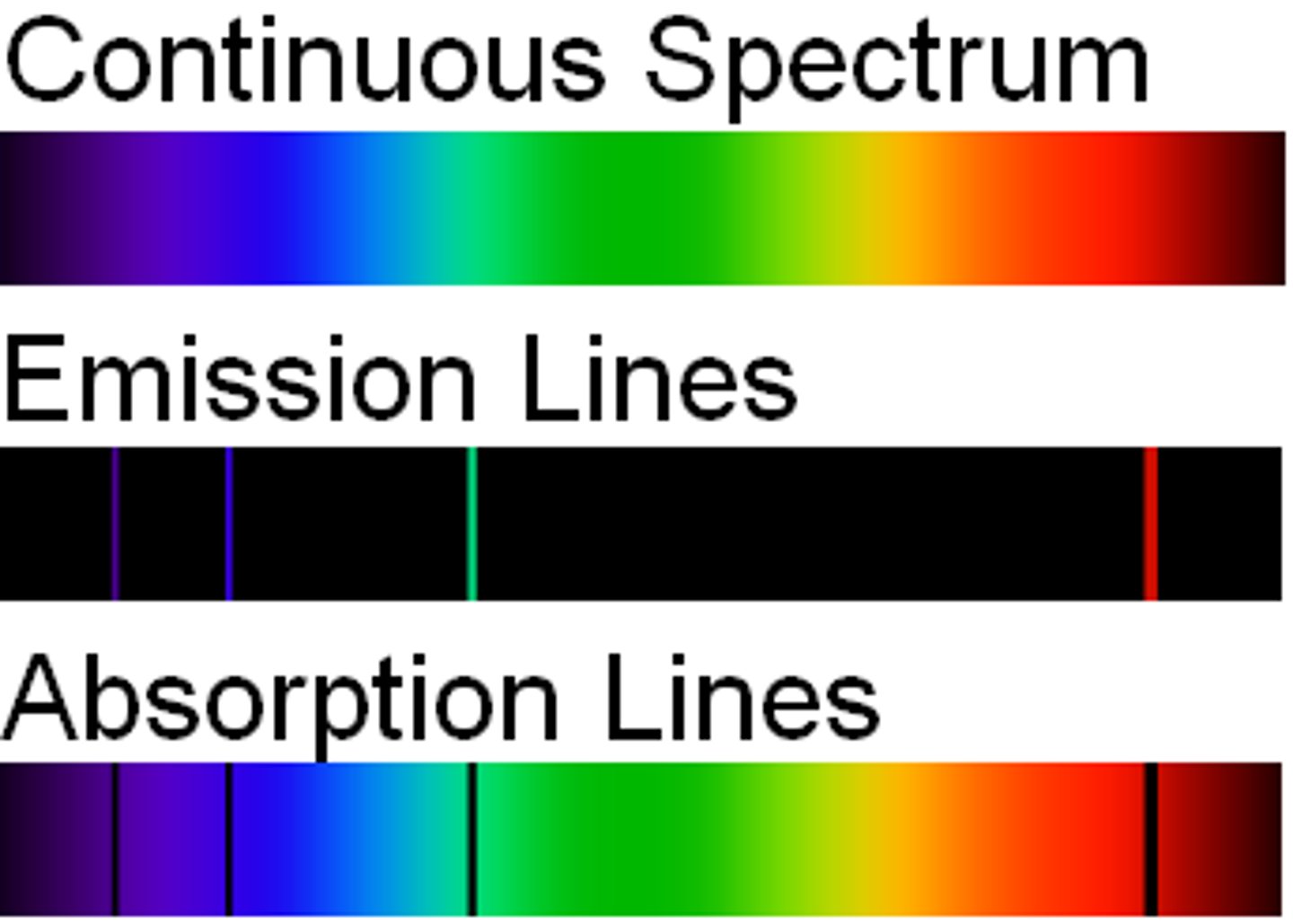
Emission spectra
Electrons transition from a higher energy level to a lower energy level
- each transition corresponds to a different wavelength of light and this corresponds to a line in the spectrum
Absorption spectra
An atom can be raised to an excited state by the absorption of a photon
When white light passes through a cool, low pressure gas it is found that light of certain wavelengths are missing
The wavelengths missing from an absorption spectrum are the same as their corresponding emission spectra of the same element
Evidence of light acting as a particle
Photoelectric effect
Evidence of light acting as a wave
Diffraction & interference of light in Young's Double-Slit experiment
The wave theory of light suggests that "Any frequency of light can give rise to photoelectric emission if the exposure time is long enough"
This is wrong because "Photoelectrons will be released immediately if the frequency is above the threshold for that metal"
The wave theory of light suggests that "The energy absorbed by each electron will increase gradually with each wave"
This is wrong because "Energy is absorbed instantaneously - photoelectrons are either emitted or not emitted after exposure to light"
The wave theory of light suggests that "The kinetic energy of the emitted electrons should increase with radiation intensity"
This is wrong because "If the intensity of the light is increased, more photoelectrons are emitted per second"
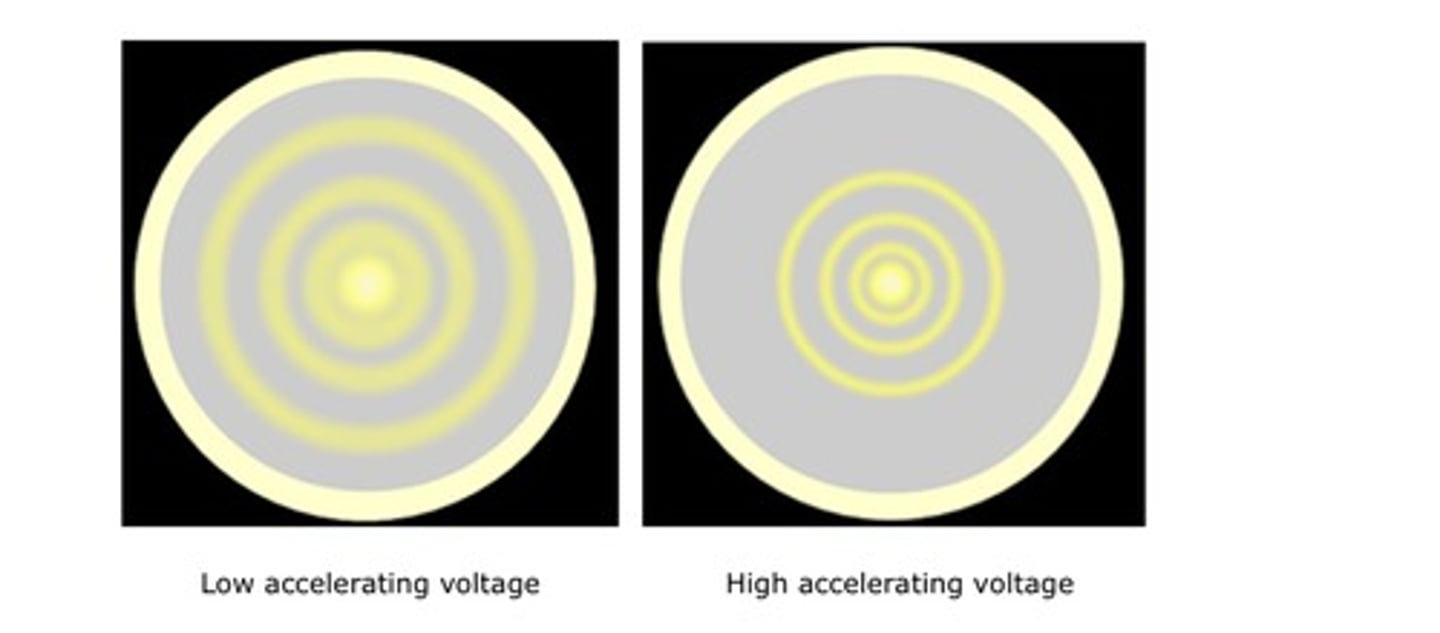
Electron diffraction
Concentric rings are observed
- a larger accelerating voltage reduces the diameter of a given ring
- a lower accelerating voltage increases the diameter of the rings