AP Biology - UNIT 2B: CELL MEMBRANE STRUCTURE & MEMBRANE TRANSPORT ASSESSMENT
1/30
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
31 Terms
(ENERGY / ATP / ENZYMES) An Introduction to Metabolism
What is ENERGY?
the capacity to do work or cause change (in chemical terms, it’s the capacity to rearrange matter)
(ENERGY / ATP / ENZYMES) An Introduction to Metabolism
ENERGY CAN’T BE _______ OR _________, BUT IT CAN ______ ____!
CREATED
DESTROYED
CHANGE FORM
(ENERGY / ATP / ENZYMES) An Introduction to Metabolism
____ ______ is released to the environment with every energy transformation.
Heat energy
(ENERGY / ATP / ENZYMES) An Introduction to Metabolism
What is KINETIC ENERGY?
energy of motion (heat, light)
(ENERGY / ATP / ENZYMES) An Introduction to Metabolism
What is POTENTIAL ENERGY?
stored (in the bonds)/positional energy (chemical)
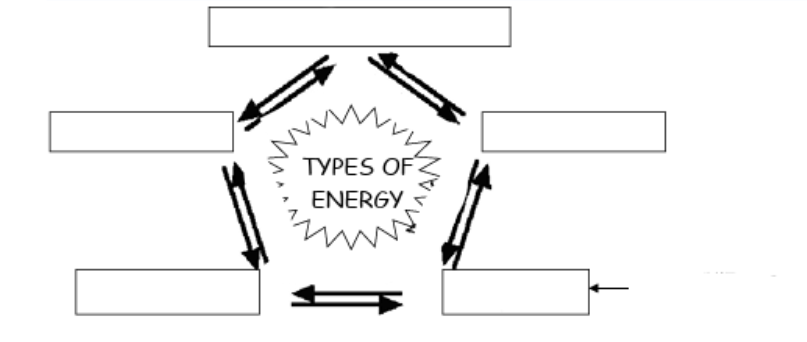
(ENERGY / ATP / ENZYMES) An Introduction to Metabolism
Fill in this chart about TYPES OF ENERGY.
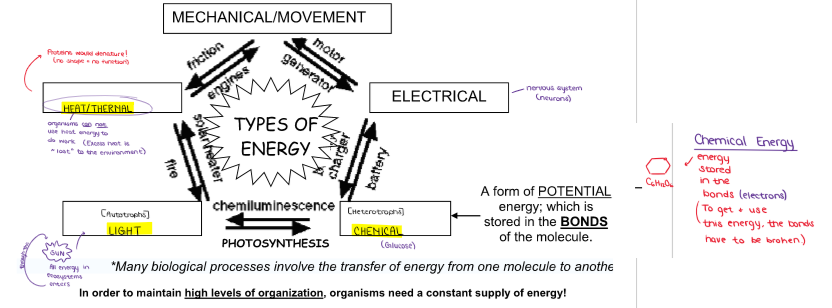
(ENERGY / ATP / ENZYMES) An Introduction to Metabolism
*Many biological processes involve the transfer of energy from one molecule to another. In order to maintain high levels of organization, organisms need a constant supply of energy! Cells need a constant supply of energy for…
Normal cell/tissue maintenance & repair
Performing the life functions (metabolism)
Growth
Excretion
Nutrition
Transport
Synthesis
Respiration
Reproduction
Regulation
(ENERGY / ATP / ENZYMES) CELLULAR METABOLISM
What is CELLULAR METABOLISM?
all chemical rxns
(ENERGY / ATP / ENZYMES) CELLULAR METABOLISM
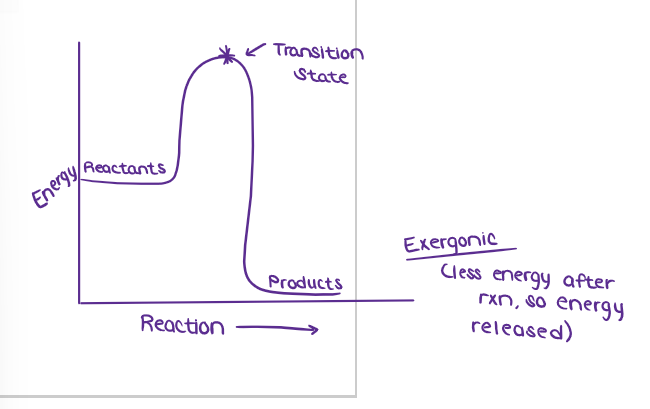
What are EXERGONIC REACTIONS?
releases energy; energy is a product
BONDS ARE BROKEN
Example: Cellular Respiration! (occurs in Mitochondria)
C6H12O6 + O2 → CO2 + H2O + ENERGY (ATP + heat)
ENERGY → Chemical energy is stored in molecules of ADENOSINE TRIPHOSPHATE (ATP) (the energy molecule of the cell)
(ENERGY / ATP / ENZYMES) CELLULAR METABOLISM
What are ENDERGONIC REACTIONS?
All synthesis rxns
requires energy; energy is a reactant
BONDS ARE FORMED
Example: Photosynthesis! (occurs in Chloroplast)
ENERGY + CO2 + H2O → C6H12O6 + O2
ENERGY → Sunlight energy (a form of kinetic energy)
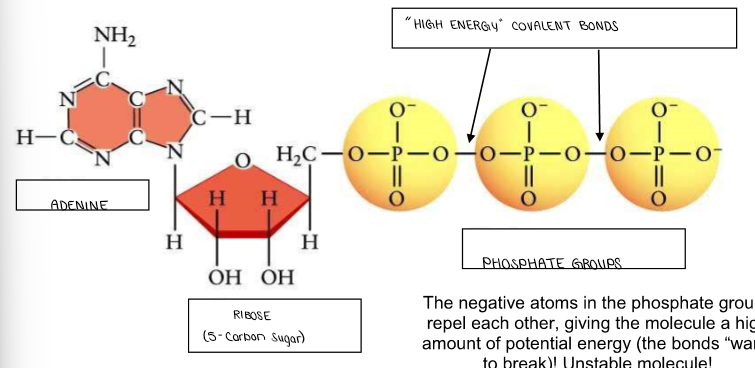
(ENERGY / ATP / ENZYMES) A CLOSER LOOK AT ATP!
ATP consists of…
ADENINE (also found in DNA/RNA) + RIBOSE SUGAR (also found in RNA) → Adenosine
3 PHOSPHATE GROUPS → Triphosphate
2 HIGH ENERY BONDS (covalent) between phosphate groups.
(High energy bonds are often indicated by ~, instead of “straight” covalent bonds)
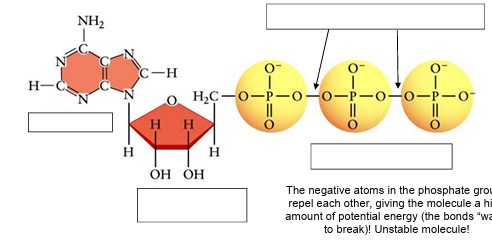
(ENERGY / ATP / ENZYMES) A CLOSER LOOK AT ATP!
Label the ATP molecule.
(ENERGY / ATP / ENZYMES) A CLOSER LOOK AT ATP!
Why is ATP an UNSTABLE molecule?
The negative atoms in the phosphate groups repel each other, giving the molecule a high amount of potential energy (the bonds “want” to break)!
high energy “pops” that get used in cells when the bond breaks
(ENERGY / ATP / ENZYMES) PHOSPHORYLATION REACTIONS
What are PHOSPHORYLATION REACTIONS?
*reactions that transfer phosphate groups, causing the transfer of energy from one molecule to the other*
“ENERGY COUPLING"
Involves the breaking of high energy bonds from ATP → EXERGONIC (That “pop” of energy can be used to do “work” in the cell.)
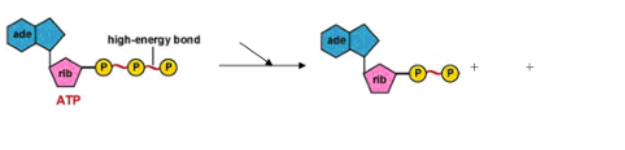
(ENERGY / ATP / ENZYMES) PHOSPHORYLATION REACTIONS
Label this DEPHOSPHORYLATION reaction.
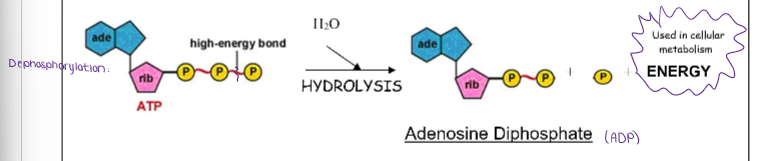
(ENERGY / ATP / ENZYMES) PHOSPHORYLATION REACTIONS
When a phosphate group that breaks off from ATP can be transferred to another molecule, making a new bond…
ENDERGONIC

(ENERGY / ATP / ENZYMES) PHOSPHORYLATION REACTIONS
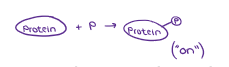
The molecule that receives a phosphate group is…
PHOSPHORYLATED
(ENERGY / ATP / ENZYMES) PHOSPHORYLATION REACTIONS
The molecule that loses a phosphate group is…
DEPHOSPHORYLATED
(ENERGY / ATP / ENZYMES) PHOSPHORYLATION REACTIONS
ATP provides the energy for 3 types of cellular work…
Chemical (like synthesis reactions: DNA synthesis, protein synthesis)
mechanical (like the movement of cilia or flagella, muscles)
transport (like movement of ions against the gradient)
Endocytosis/Exocytosis
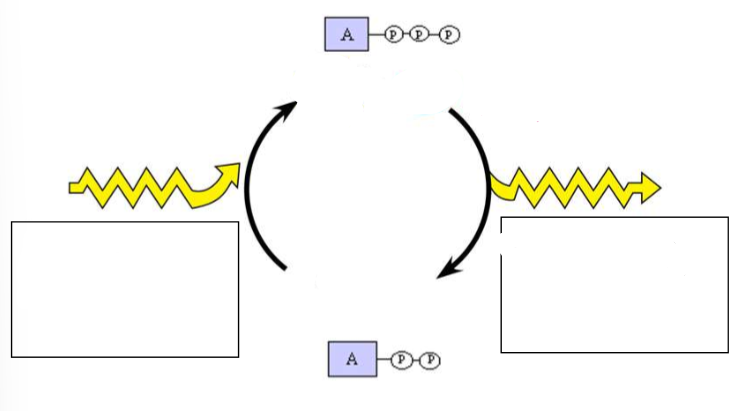
(ENERGY / ATP / ENZYMES) ATP/ADP CYCLE
Label the diagram.
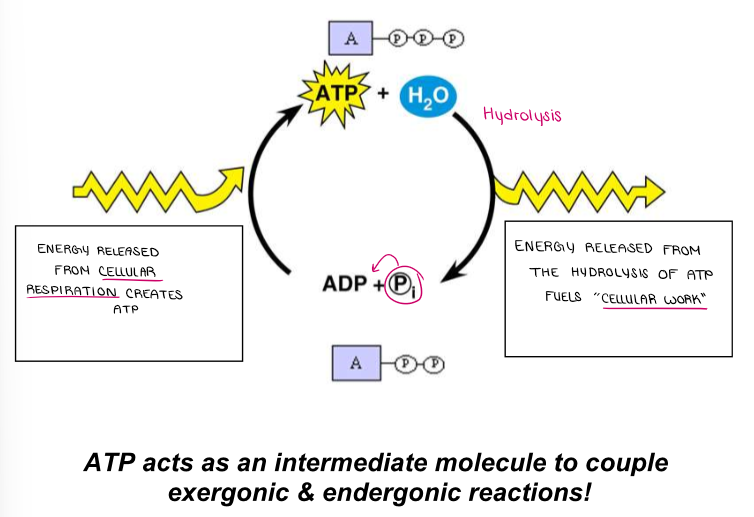
(ENERGY / ATP / ENZYMES) Why can’t the cell just use the chemical energy stored in glucose? (Why did an intricate multi-step pathway evolve to create ATP?)
It would release too much energy at once! Metabolic pathways evolved to slowly release and transfer the energy without damaging the cell.
ATP holds & releases smaller, more manageable amounts of energy so none is wasted. (Energy efficiency)
(ENERGY / ATP / ENZYMES) Why can’t the cell just use the chemical energy stored in glucose? (Why did an intricate multi-step pathway evolve to create ATP?)
Compare GLUCOSE and ATP.
GLUCOSE:
lots of energy
STABLE for travel in bloodstream
ATP:
smaller amounts of energy
only utilized INSIDE CELLS
unstable (easy to break bonds)
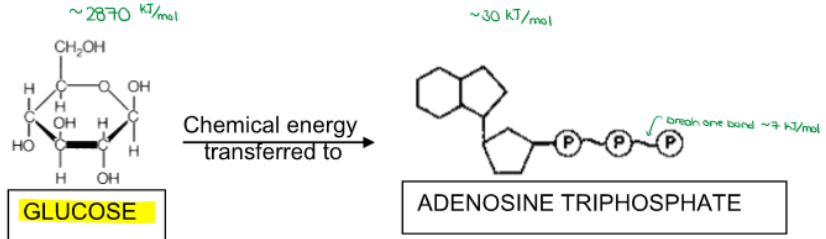
(ENERGY / ATP / ENZYMES) Why can’t the cell just use the chemical energy stored in glucose? (Why did an intricate multi-step pathway evolve to create ATP?)
1 glucose is equivalent to __ ATP!
32
(ENERGY / ATP / ENZYMES) ENZYMES: Nature’s Catalysis for Life!
What are ENZYMES?
Enzymes are specialized proteins that act as biological catalysts, starting and speeding up chemical reactions necessary for life.
They are required for reactions to occur, allowing processes like digestion, energy production, and DNA replication to happen efficiently.
Each enzyme is highly specific with a specific shape/active site, binding to a particular substrate and facilitating a unique reaction making them essential for maintaining the body’s metabolism and homeostasis.
Often (but not always) have “-ASE” endings
Enzymes are needed in very small amounts because they are recycled/reused (unchanged) after the reaction occurs
they are not interchangeable!
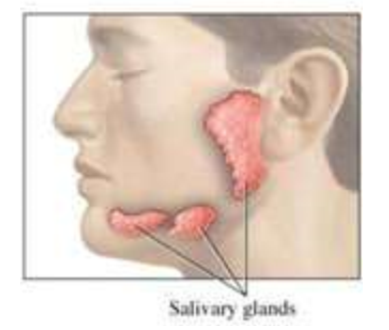
(ENERGY / ATP / ENZYMES) ENZYMES: Nature’s Catalysis for Life!
Where do enzymes work?
Enzymes can’t be consumed in our diet…they are synthesized in cells. Most enzymes work inside the cell (anywhere from 1000 - 4000 in animal cells), but some get secreted (sent out) and work outside the cell…like digestive enzymes inside the stomach or mouth.
(ENERGY / ATP / ENZYMES) ENZYMES: Nature’s Catalysis for Life!
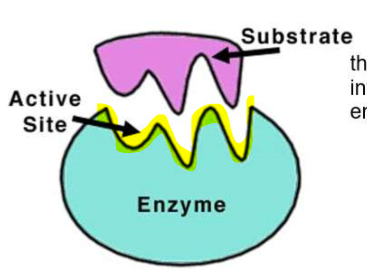
What is the ACTIVE SITE?
ACTIVE SITE the site where the chemical reaction occurs
The substrate enters the active site - the shape is very important!
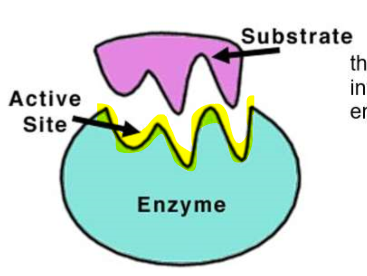
(ENERGY / ATP / ENZYMES) ENZYMES: Nature’s Catalysis for Life!
What is the SUBSTRATE?
the substances that interact with the enzyme (reactants)
(ENERGY / ATP / ENZYMES) Enzyme Specificity
Explain enzyme specificity.
Every reaction has its own specific enzyme. They are not interchangeable.
Enzymes are not included in the chemical reaction, but can be written over the yield sign because:
they do not get changed in any way
they are recycled (used over and over again)

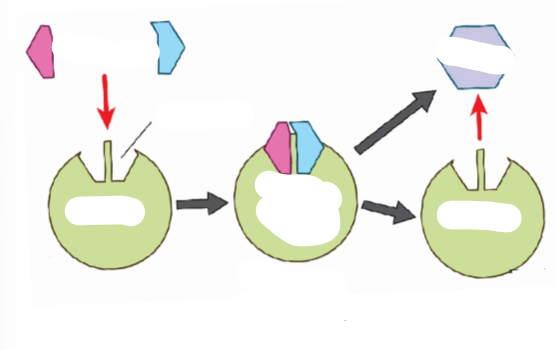
(ENERGY / ATP / ENZYMES) Enzyme Specificity
Label the process of an enzyme.
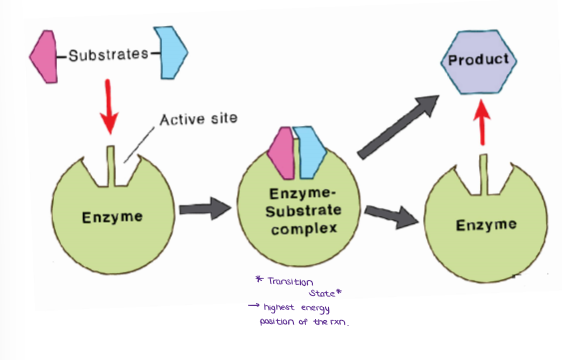
(ENERGY / ATP / ENZYMES) Enzyme Specificity
What is the TRANSITION STATE?
highest energy position of the rxn
(ENERGY / ATP / ENZYMES) Enzyme Specificity
Draw the graph of an enzyme reaction.