Atomic Spectra and Quantum Mechanics Overview
1/21
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
22 Terms
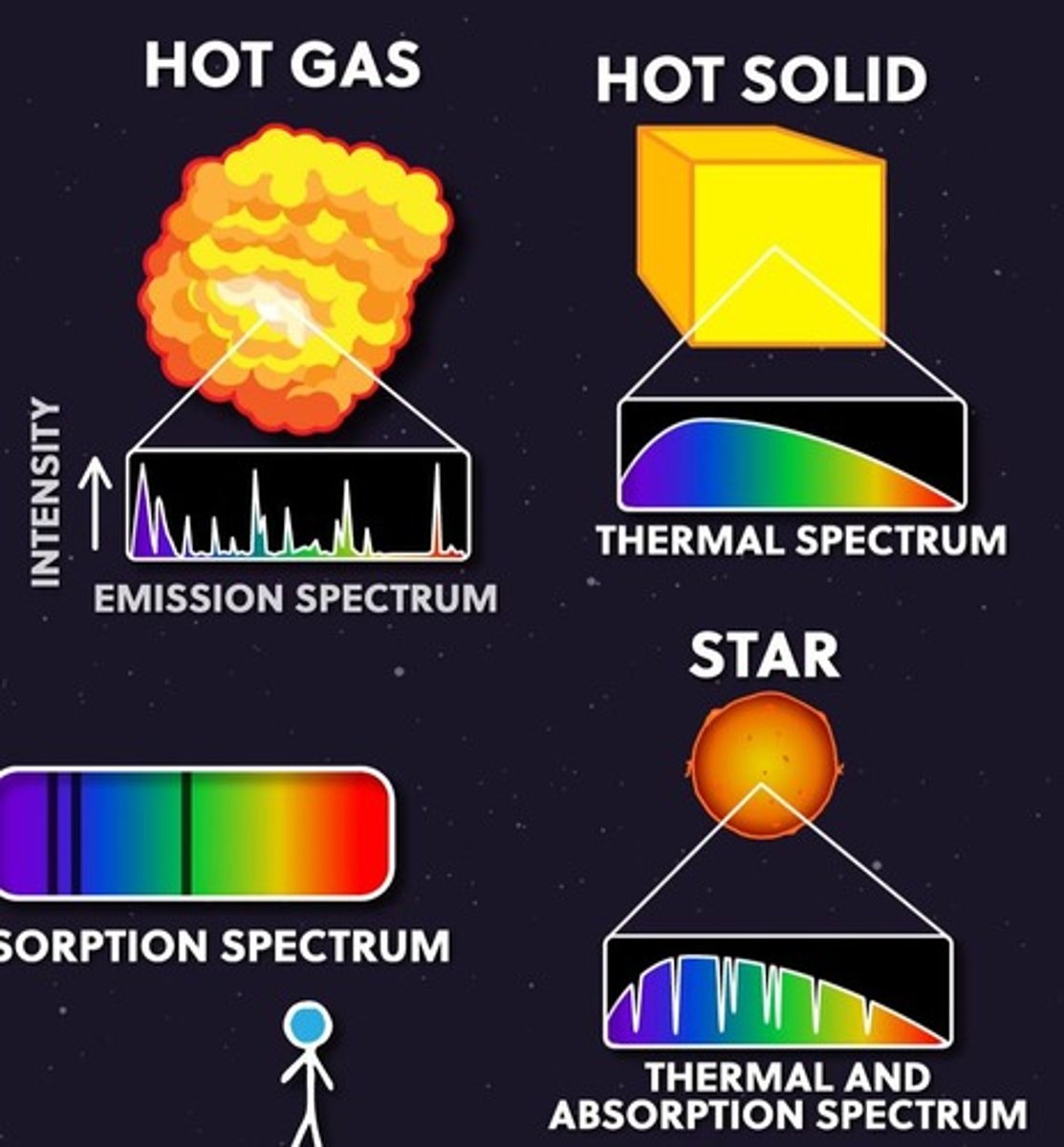
Atomic Spectrum
Characteristic colors emitted by heated substances.
Emission Spectrum
Light emitted by substances at specific wavelengths.
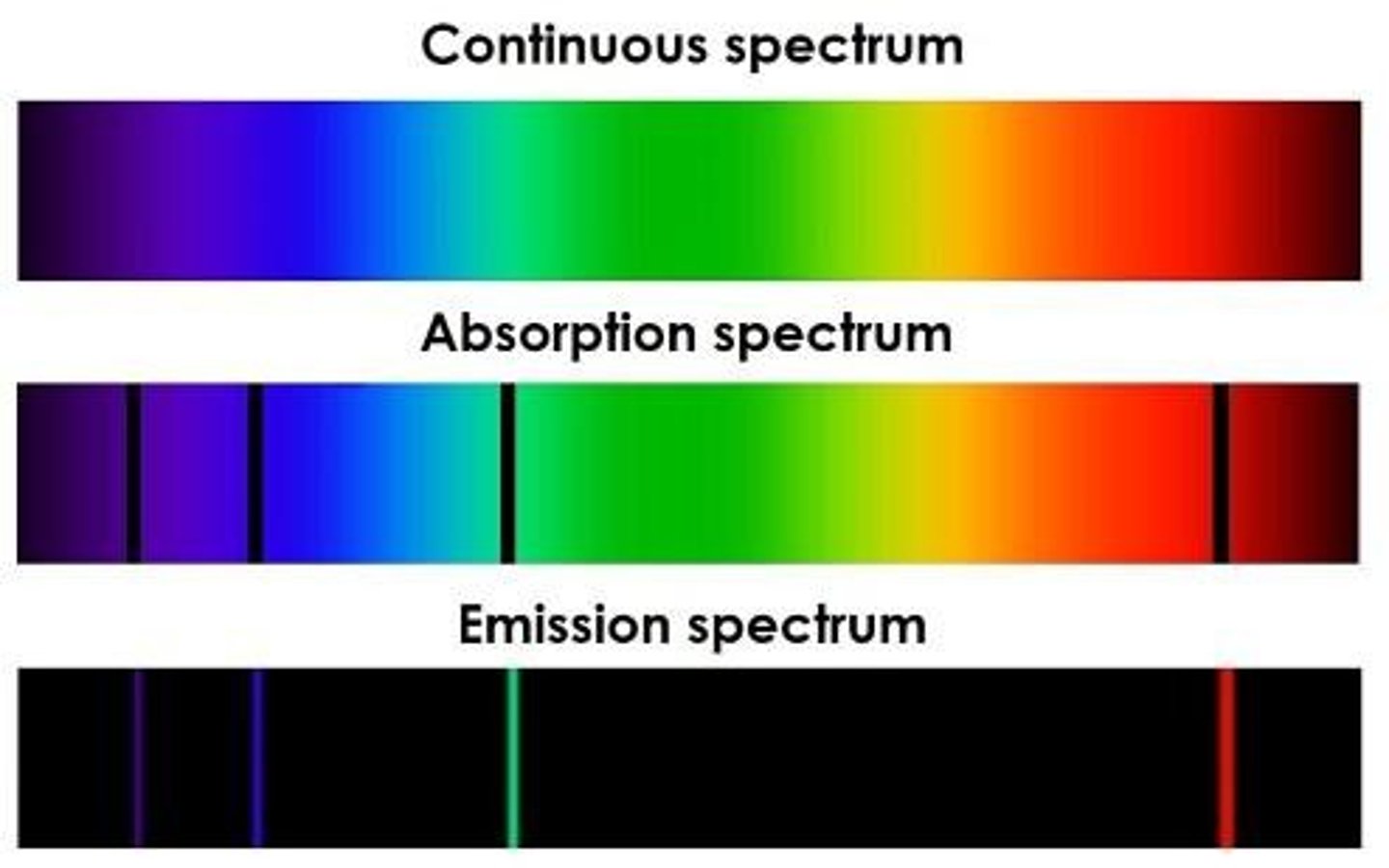
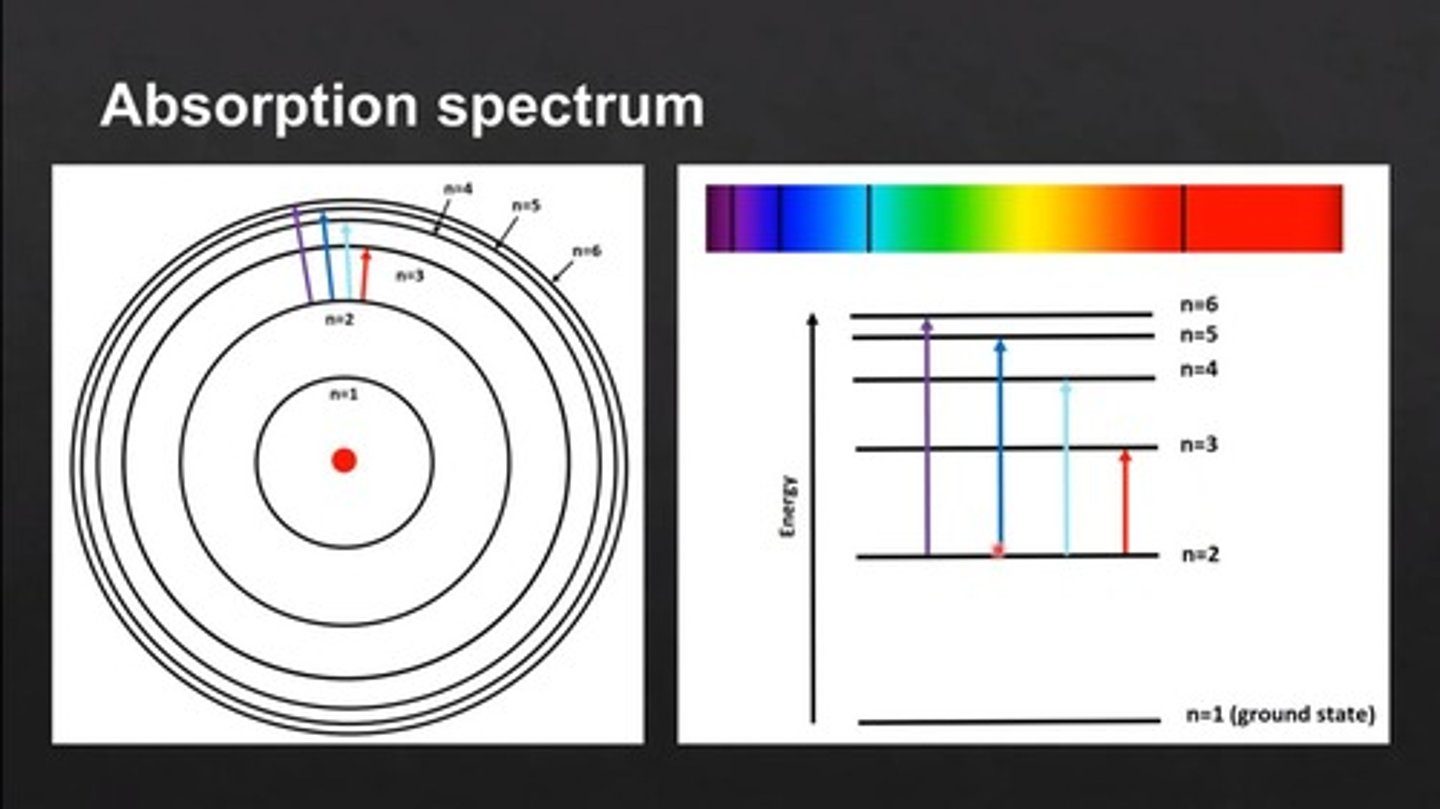
Absorption Spectrum
Wavelengths absorbed by a substance, creating dark lines.
Planck's Constant
h = 6.626 x 10^-34 J s, quantizes light energy.
Quantum Mechanics
Study of particles exhibiting wave-particle duality.
Bohr's Model
Electrons orbit nucleus in quantized energy levels.
Photon
Light quantum with energy E = hf.
Blackbody Radiator
Ideal emitter of continuous electromagnetic radiation.
Wavelength
Distance between successive peaks of a wave.
Ultraviolet Light
High-energy light that can ionize organic molecules.
Melanin
Dark pigment produced in response to sunlight.
Sunscreen Ingredients
Chemical filters like oxybenzone protect against UV.
Tungsten Filament
Source of white light containing all colors.
Hydrogen Spectrum
Distinct emission lines from hydrogen atom.
Electromagnetic Waves
Radiation emitted by charged particles.
Energy Levels
Specific orbits where electrons can exist.
Ionization
Process of removing electrons from atoms.
Sunburn
Skin damage from excessive UV exposure.
Chemical Filters
Substances in sunscreens that absorb UV radiation.
Discrete Chunks
Light energy absorbed or emitted in quantized amounts.
Solar System Model
Outdated analogy for electron arrangement in atoms.
Northern Europeans
Typically have less melanin, lighter skin.