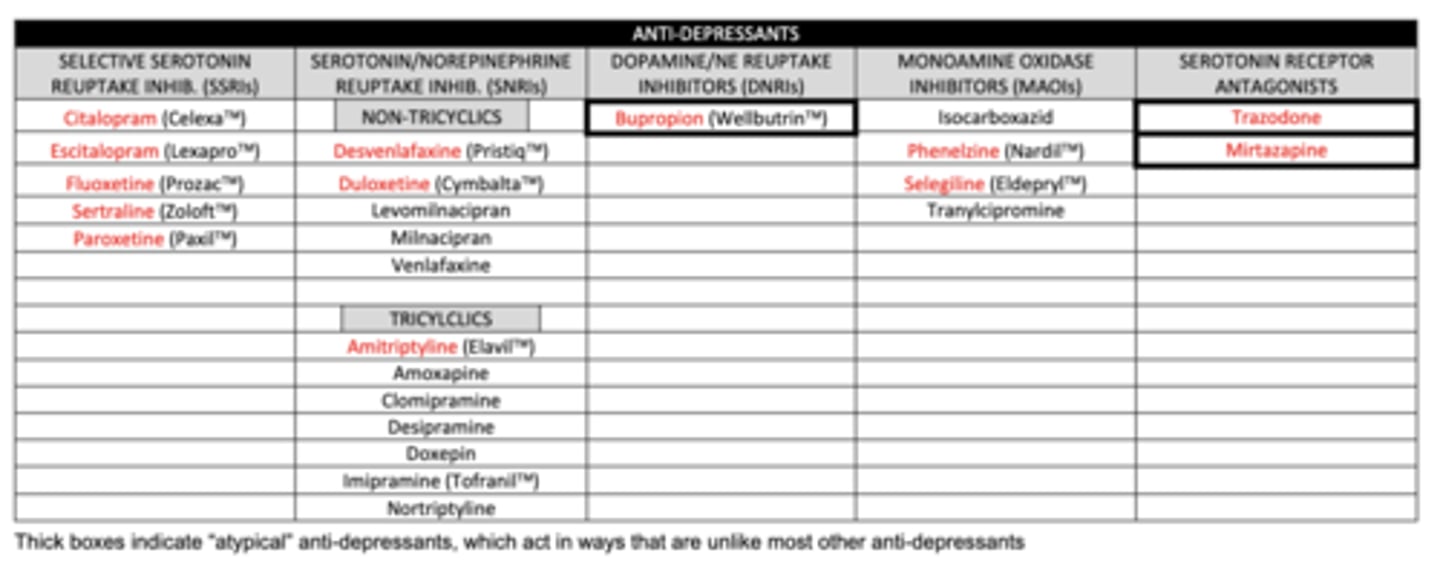
Anti-Depressants
1/46
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
47 Terms
most neurotransmitters (promote/inhibit) synaptic signaling
promote
this means that most neurotransmitters are stimulatory rather than inhibitory
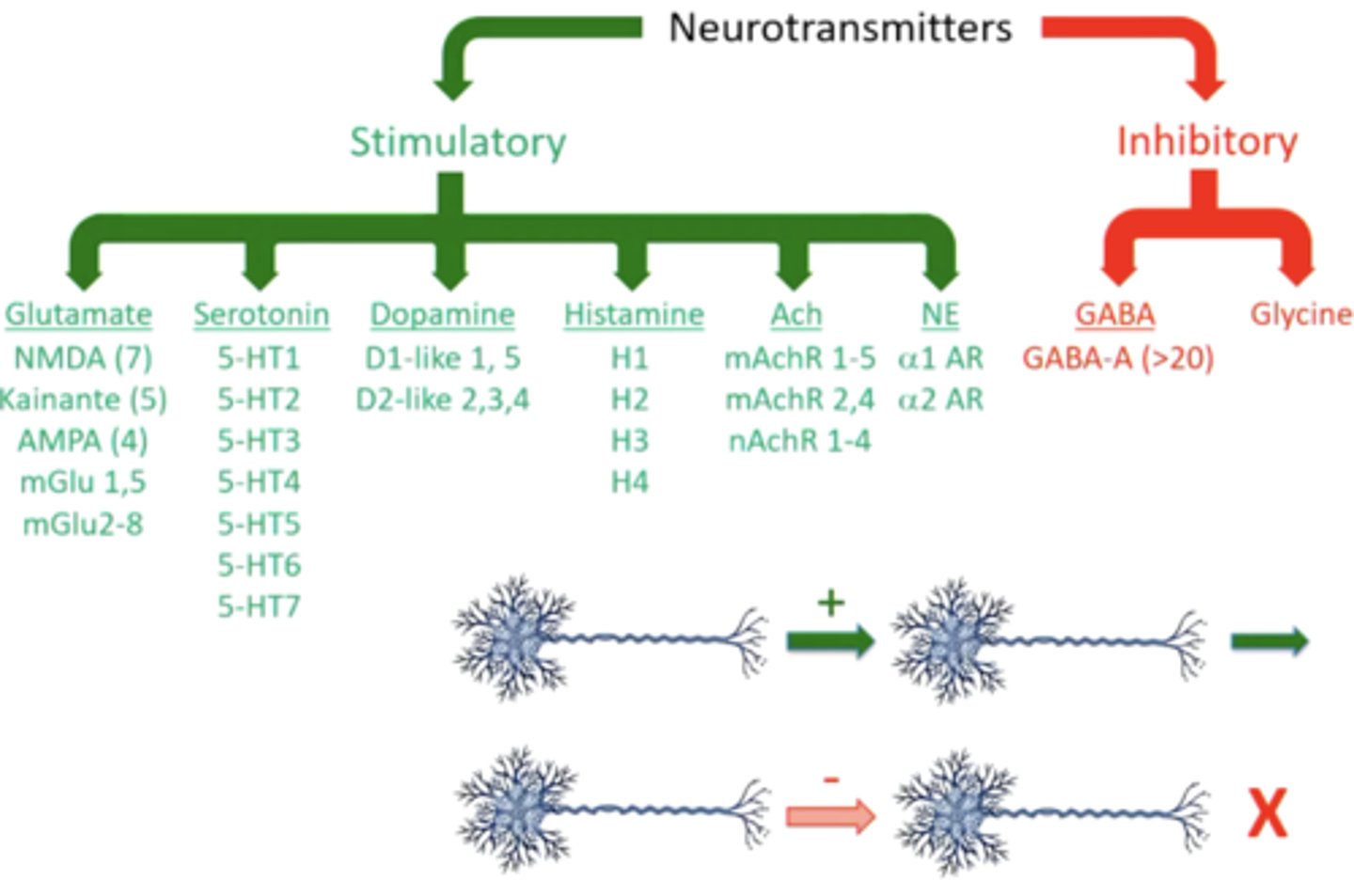
what neurotransmitter is the primary inhibitory NT?
GABA
what are the 3 neurotransmitters that are most important in terms of anti-depressants
1. serotonin
2. dopamine
3. norepinephrine
note these are all stimulatory
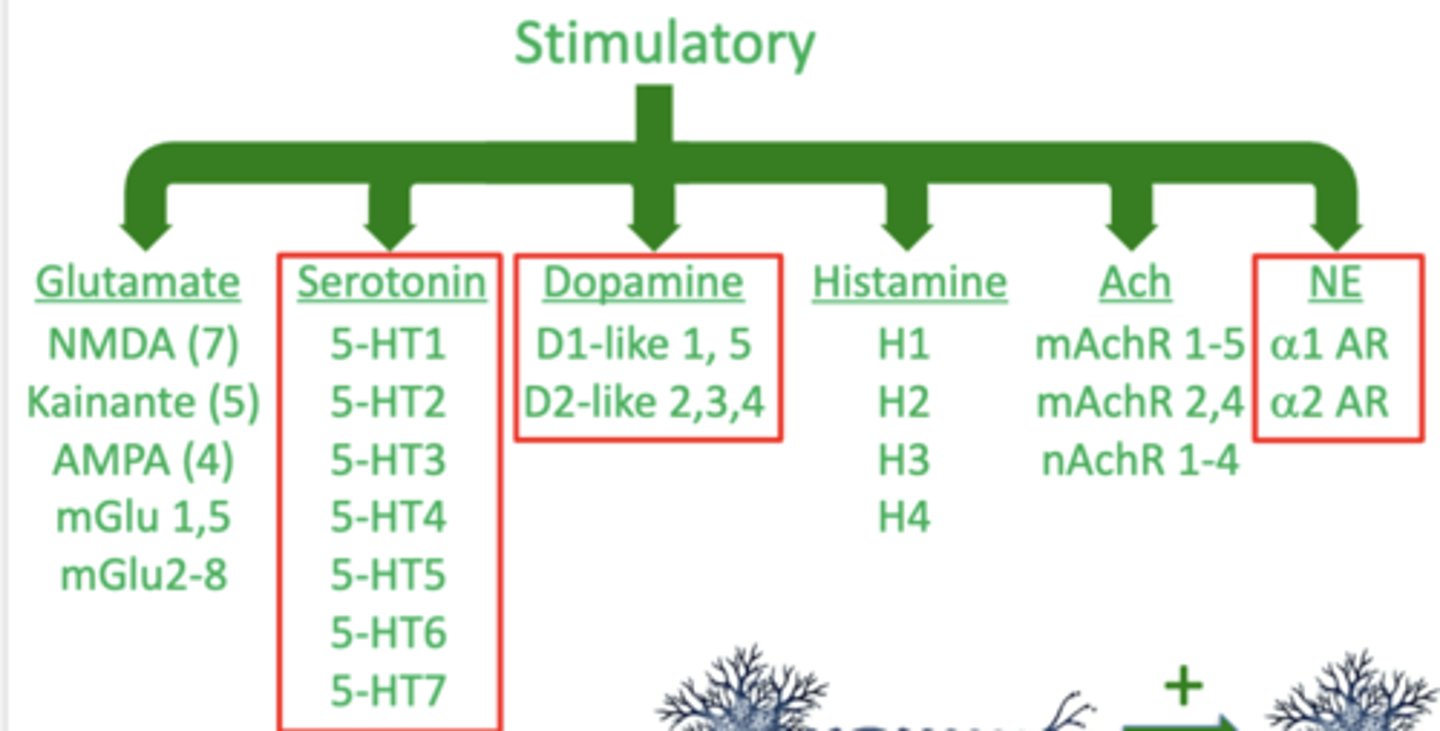
what is the monoamine hypothesis of depression?
suggests that depression is caused by a reduction in signaling by one or more of the monoamines
what are the 3 monoamines?
1. serotonin
2. dopamine
3. norepinephrine
these all have one amine (NH2) group
what enzyme is important in the degradation of monoamines?
monoamine oxidase
what evidence is there to support the monoamine hypothesis?
1. patients taking iproniazid to treat tuberculosis caused inhibition of monoamine oxidase, resulting in improved mood and energy
2. patients taking reserpine to control BP resulted in depletion of monoamines (norepinephrine, specifically), causing depression and vasodilation
T/F: currently, all anti-depressants affect the activity of monoamine neurotransmitters in some way
true
briefly describe the basic mechanism of how monoamine synapses work:
1. when depolarization occurs, the neuron fires and neurotransmitter is released
2. once signaling is over, a neurotransmitter transporter removes the neurotransmitter from the synapse, and brings it back into the presynaptic neuron
3. neurotransmitter can either be recycled and used again, or is degraded by monoamine oxidase
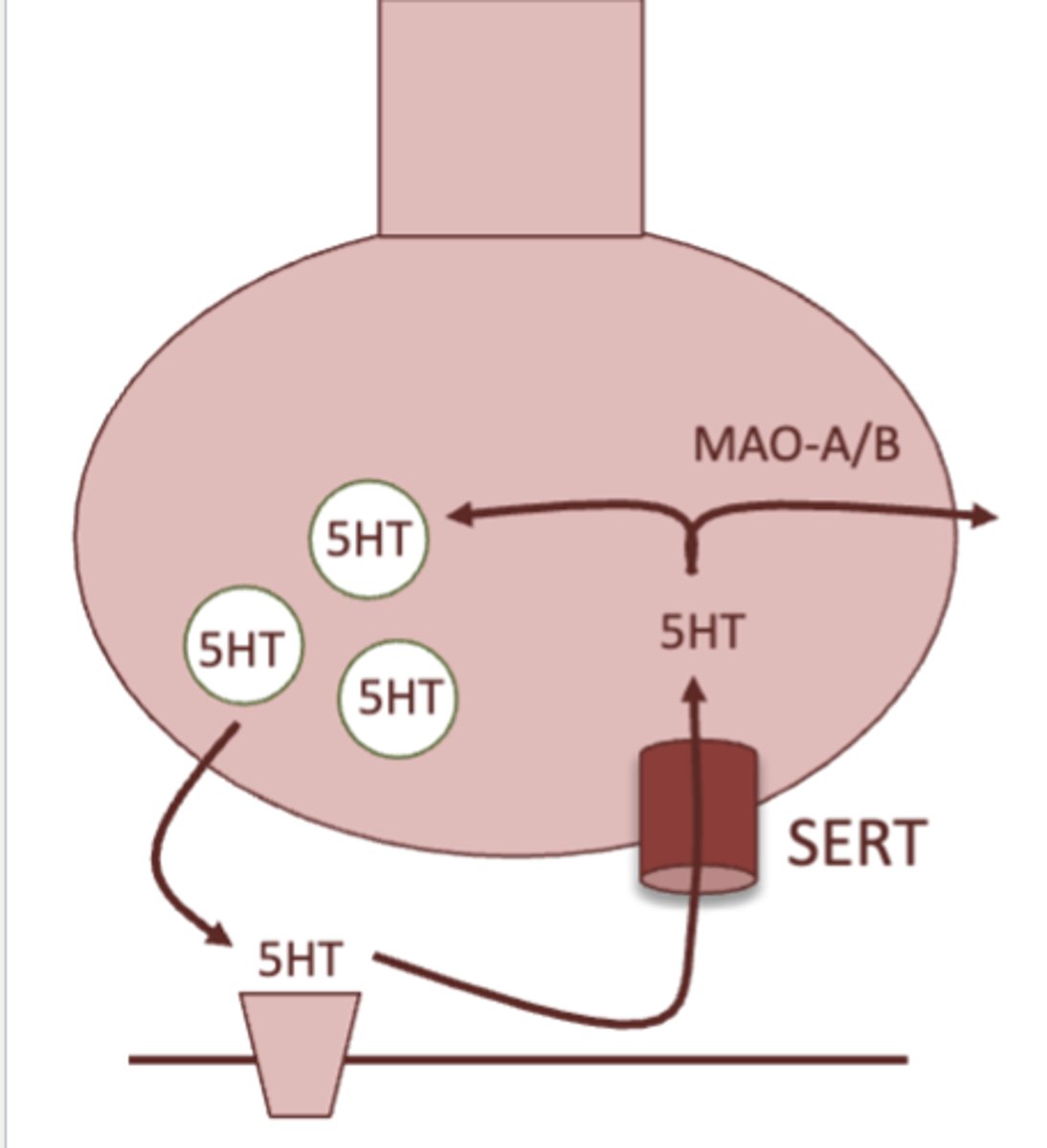
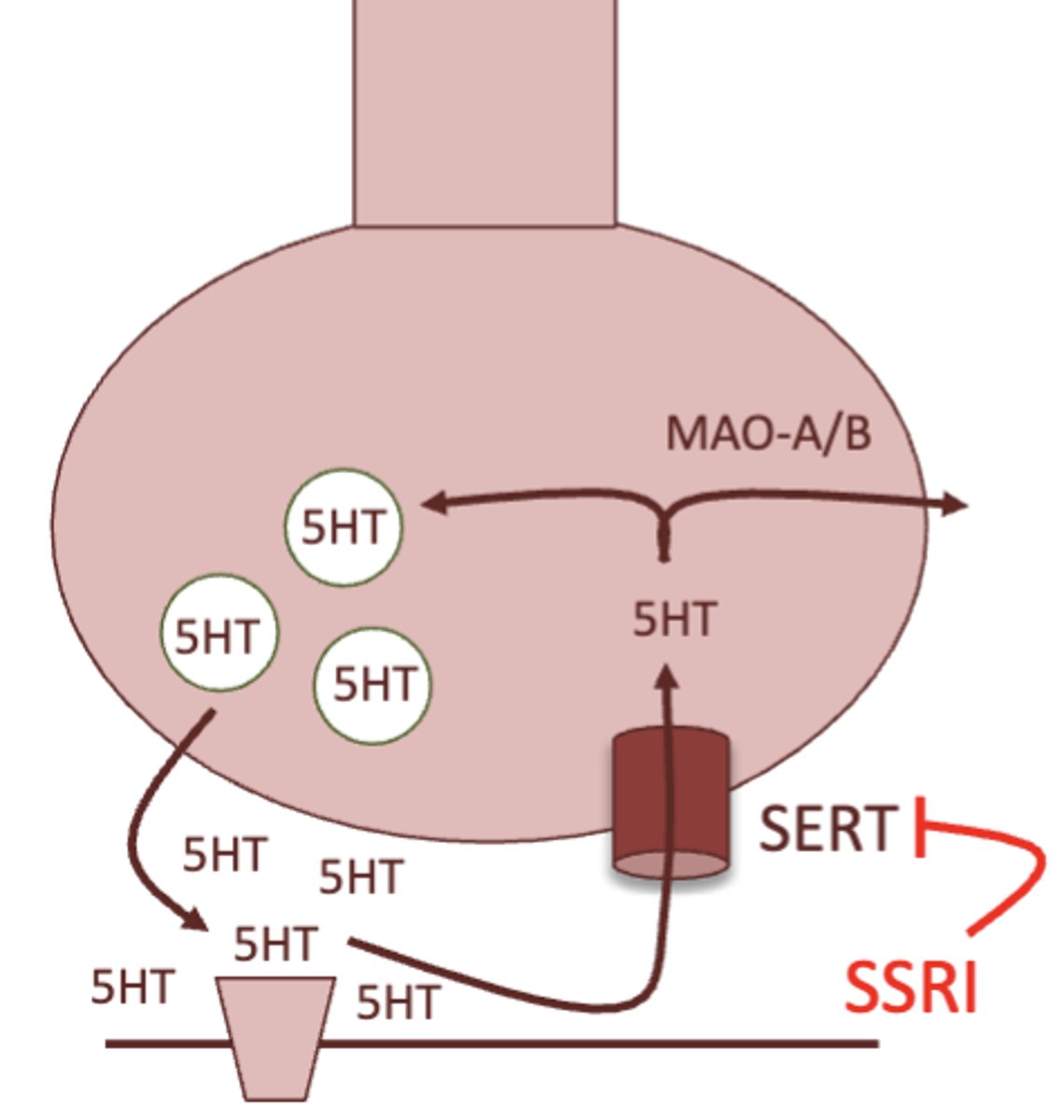
what is the MOA of SSRI's?
inhibition of the serotonin transporter, therefore increasing serotonin signaling
SSRI stands for selective serotonin reuptake inhibitor
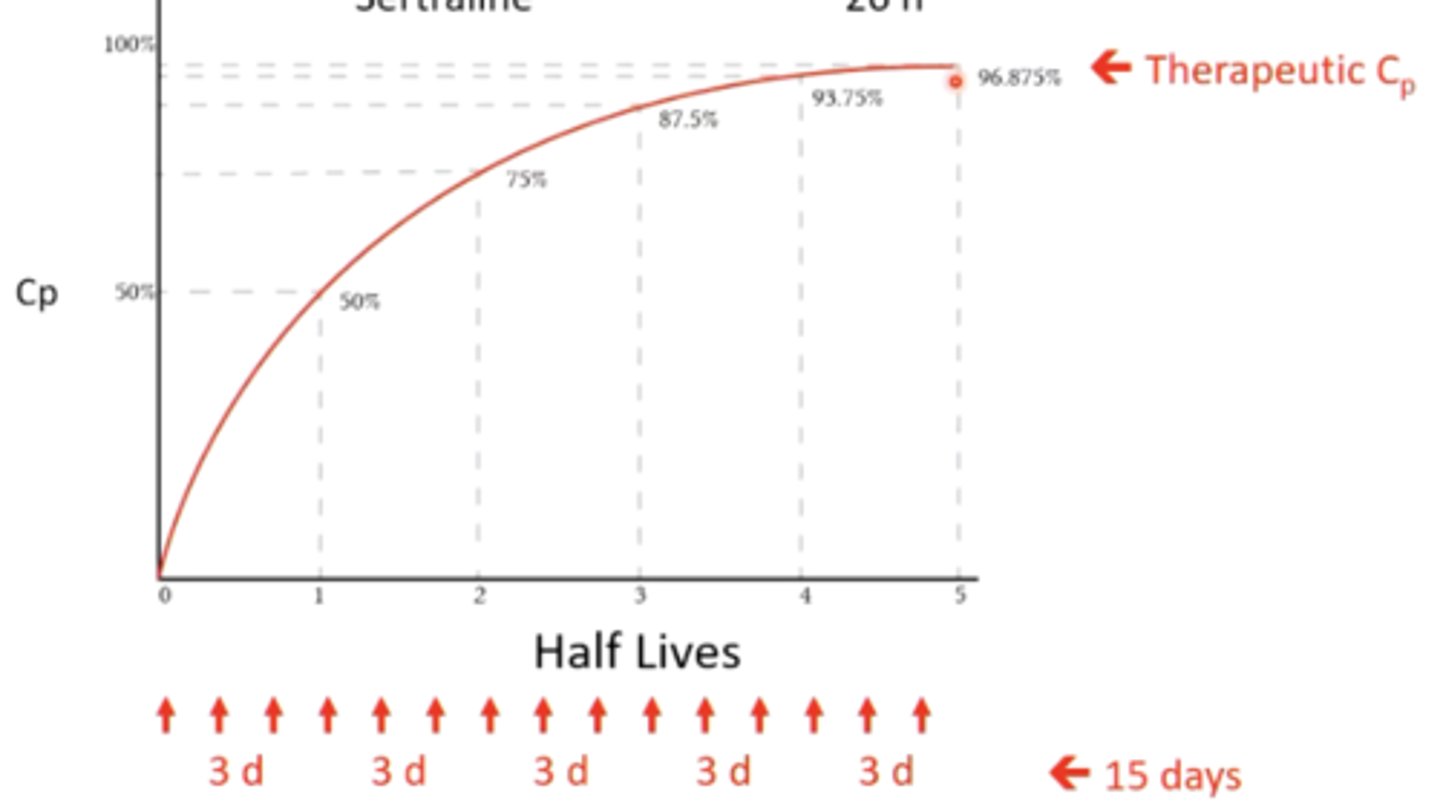
describe how the pharmacokinetics (specifically the half life) of SSRI's can be an issue:
they have long half-lives, which requires longer times to achieve therapeutic levels
(usually takes 4-5 half lives in order to reach this level)
for example, fluoxetine has a 3 day half life. It could take 15 days (3 x 5 half lives) for this drug to reach therapeutic levels in a level - will not be an immediate result
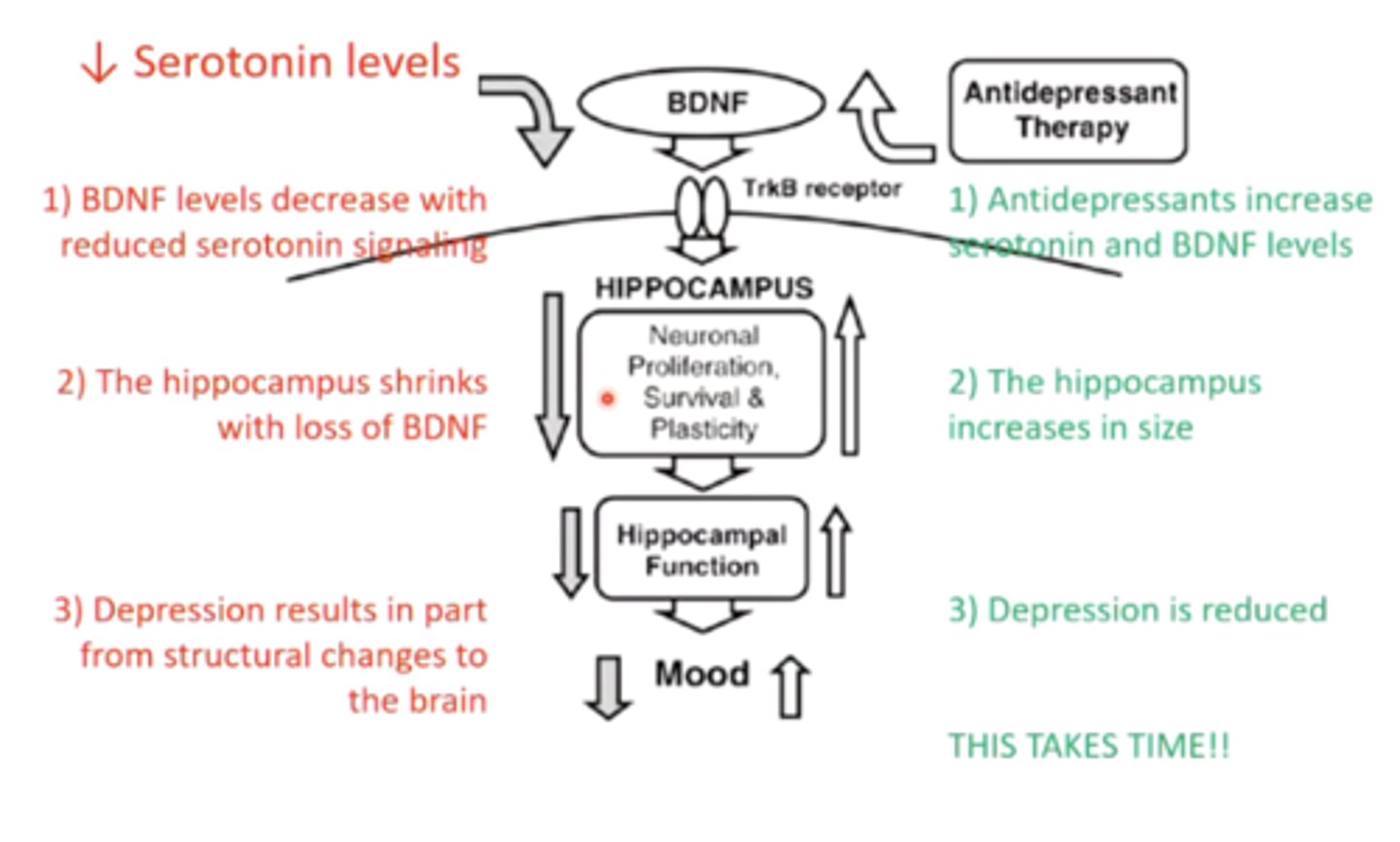
describe how the pharmacokinetics (specifically brain regeneration) of SSRI's can be an issue:
in depression, the hippocampus shrinks due to decreased BDNF levels with reduced serotonin signaling
SSRI's may take a while to work because it takes time for the hippocampus to regenerate neurons and increase BDNF and serotonin signaling
what is one of the main toxicities of SSRIs/SNRIs (in general)?
sexual dysfunction/loss of libido
what is a toxicity associated with citalopram?
prolonged QT --> increases risk for torsade de pointes
what is a toxicity associated with sertraline?
diarrhea
what SSRI should you take with caution during pregnancy?
paroxetine
it is pregnancy category D, and the other SSRIs are category C
what SSRI should you take with caution if a patient is taking a lot of other medications for other conditions?
fluoxetine and paroxetine
these inhibit CYP2D6, which is responsible for the metabolism of many drugs
taking fluoxetine or paroxetine in the presence of other drugs can increase risk for toxicities of the drugs
if drug interactions are of concern (for example, if a patient is taking a lot of different medications), what SSRI's should you consider?
citalopram and escitaprolam
what is the MOA of SNRI's?
inhibition of the serotonin and norepinephrine transporters, therefore increasing serotonin and norepinephrine signaling
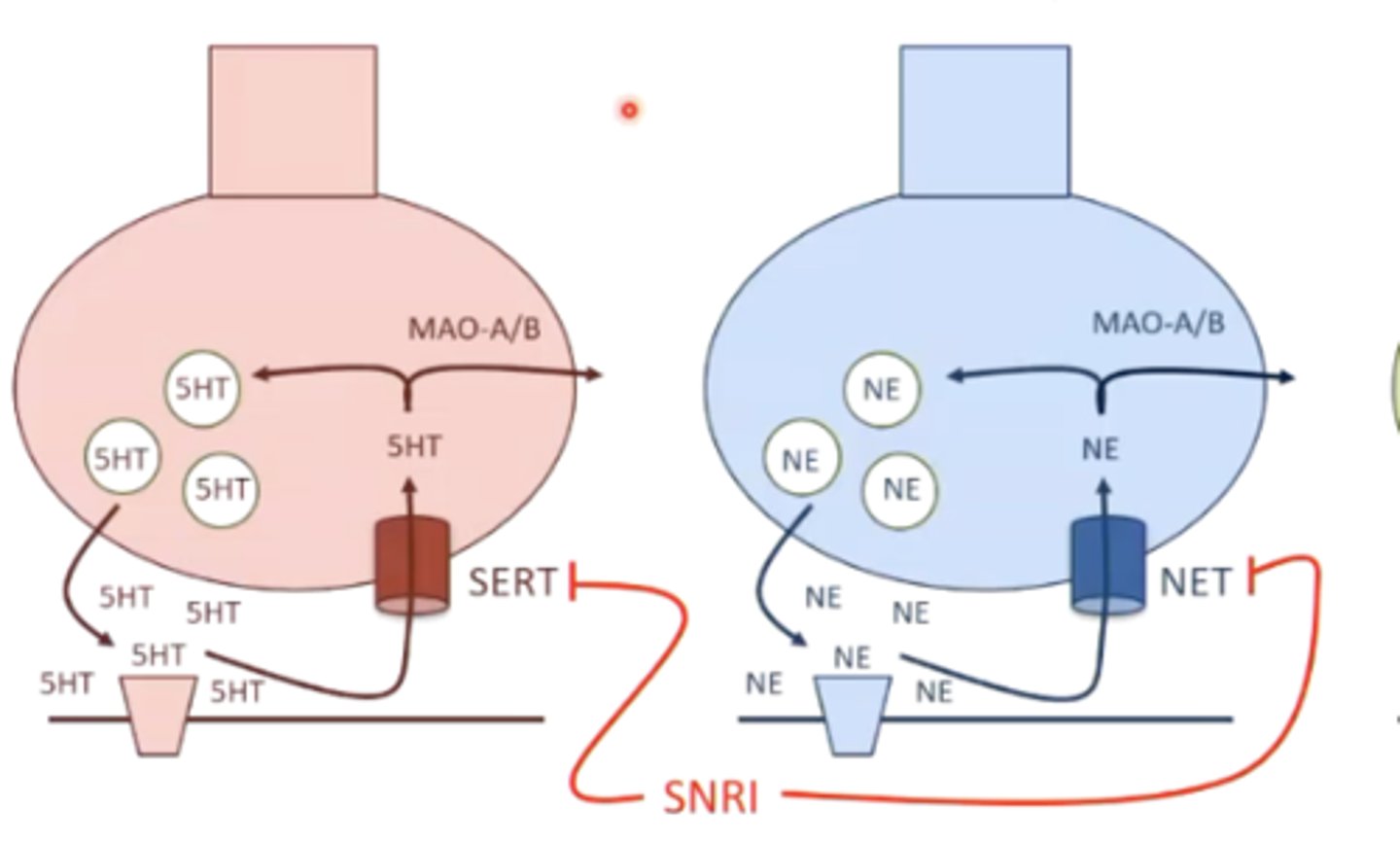
what SNRI should you take with caution if a patient is taking a lot of other medications for other conditions?
duloxetine
this inhibits CYP2D6, which is responsible for the metabolism of many drugs
taking duloxetine in the presence of other drugs can increase risk for toxicities of the drugs
what are some toxicities associated with tricyclic SNRIs? (3)
1. sedation due to H1 (histamine) antagonism in the CNS
2. orthostatic hypotension due to a1 antagonism in vascular smooth muscle
3. anti-cholinergic effects due to M1-5 antagonism in various tissues
why are tricyclic SNRIs less commonly used than non-tricyclic SNRIs?
because of the potential toxicities they produce (sedation, orthostatic hypotension, anti-cholinergic effects)
what is the MOA of bupropion?
inhibition of the dopamine transporter, therefore increasing dopamine signaling
it may also inhibit the norepinephrine transporter/reuptake, but not as much as dopamine
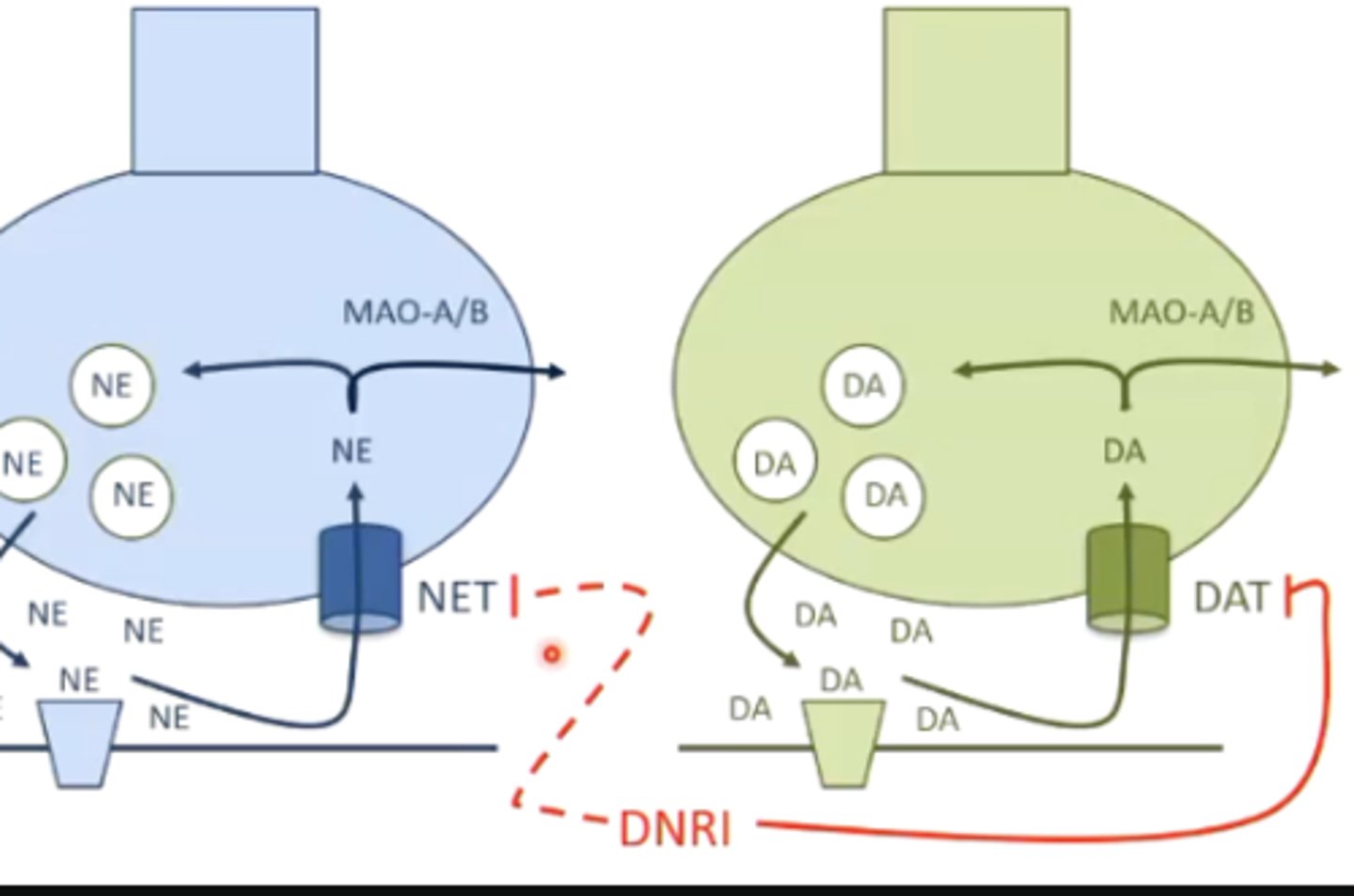
what toxicities are associated with bupropion?
increased risk of seizures
what is an advantage to taking bupropion over SSRIs and SNRIs, in terms of toxicities?
sexual dysfunction is not a potential side effect of the drug (because bupropion does not involve serotonin reuptake)
bupropion could also be used for smoking cessation
what is the MOA of monoamine oxidase inhibitors?
inhibits degradation of the monoamines by inhibiting monoamine oxidase
this indirectly increases neurotransmitter levels
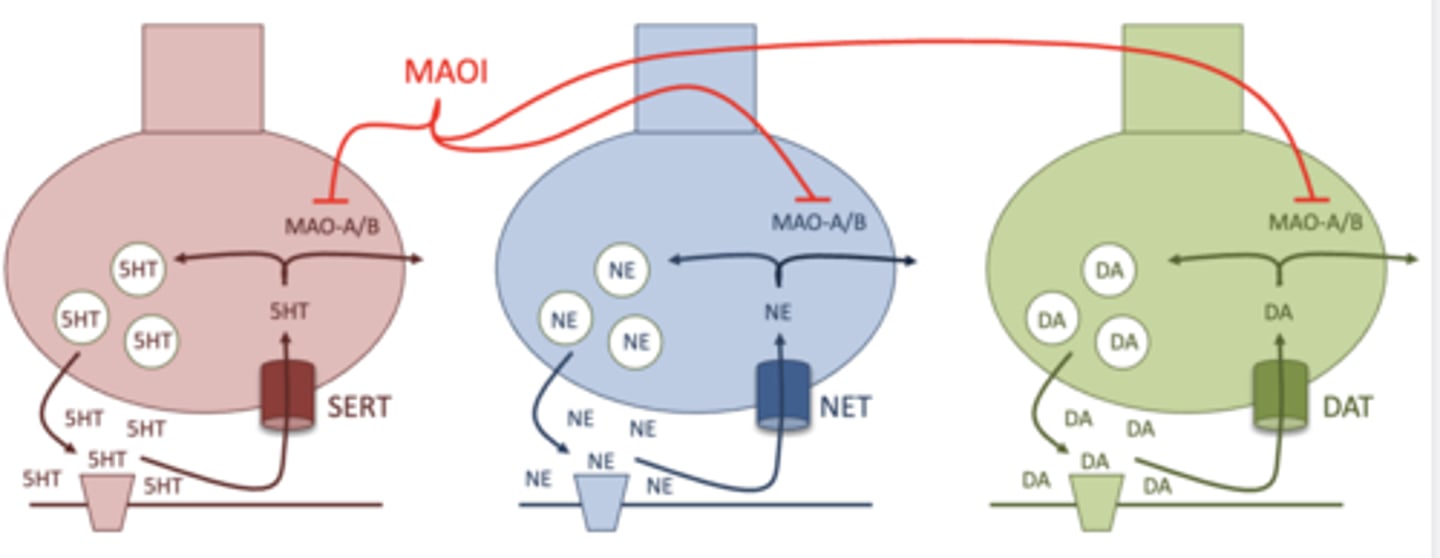
what is the difference between monoamine oxidase A (MAO-A) and monoamine oxidase B (MAO-B)?
MAO-A: degrades all of the monoamines, and is found in the brain and the gut
MAO-B: only degrades dopamine, and is found only in the brain
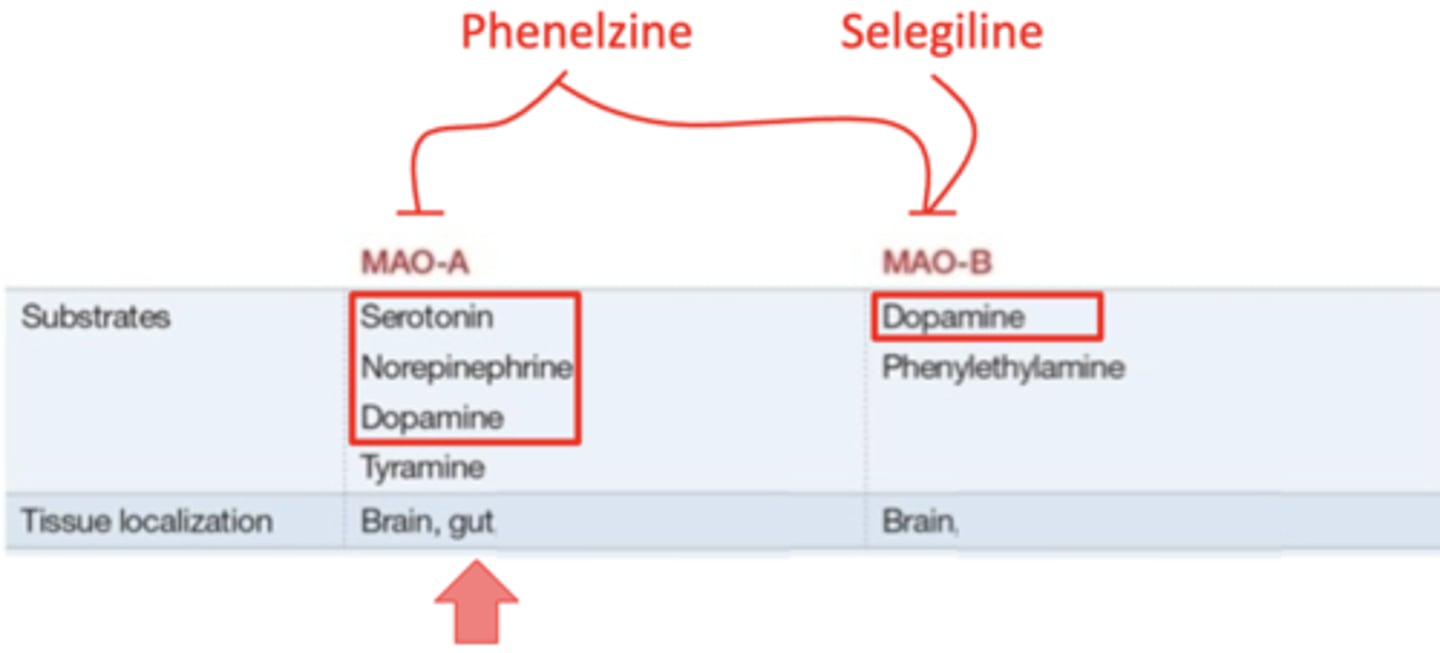
what monoamine oxidase inhibitor affects both MAO-A and MAO-B?
phenelzine
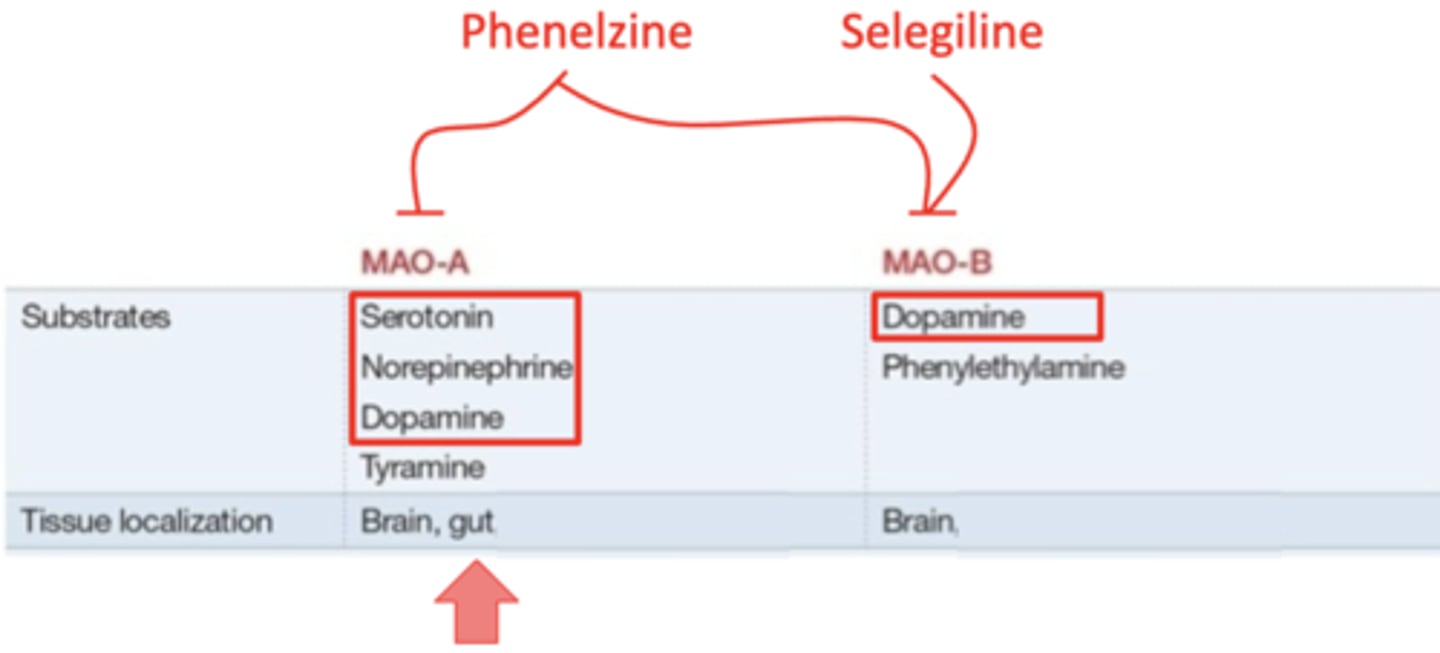
what monoamine oxidase inhibitor affects only MAO-B?
selegiline
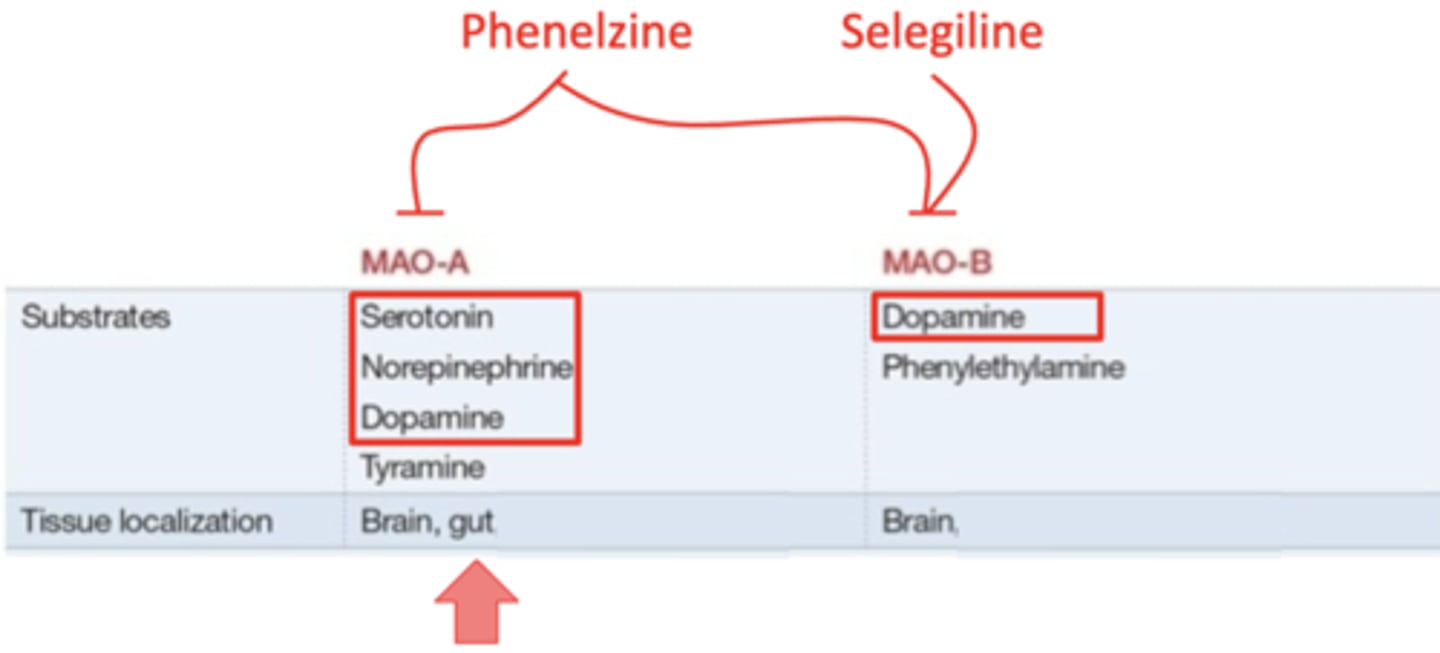
MAO (A/B) inhibitors increase absorption of tyramine in the food we eat. why is this bad?
MAO-A inhibitors
increased tyramine absorption can lead to a hypertensive crisis by increasing norepinephrine levels
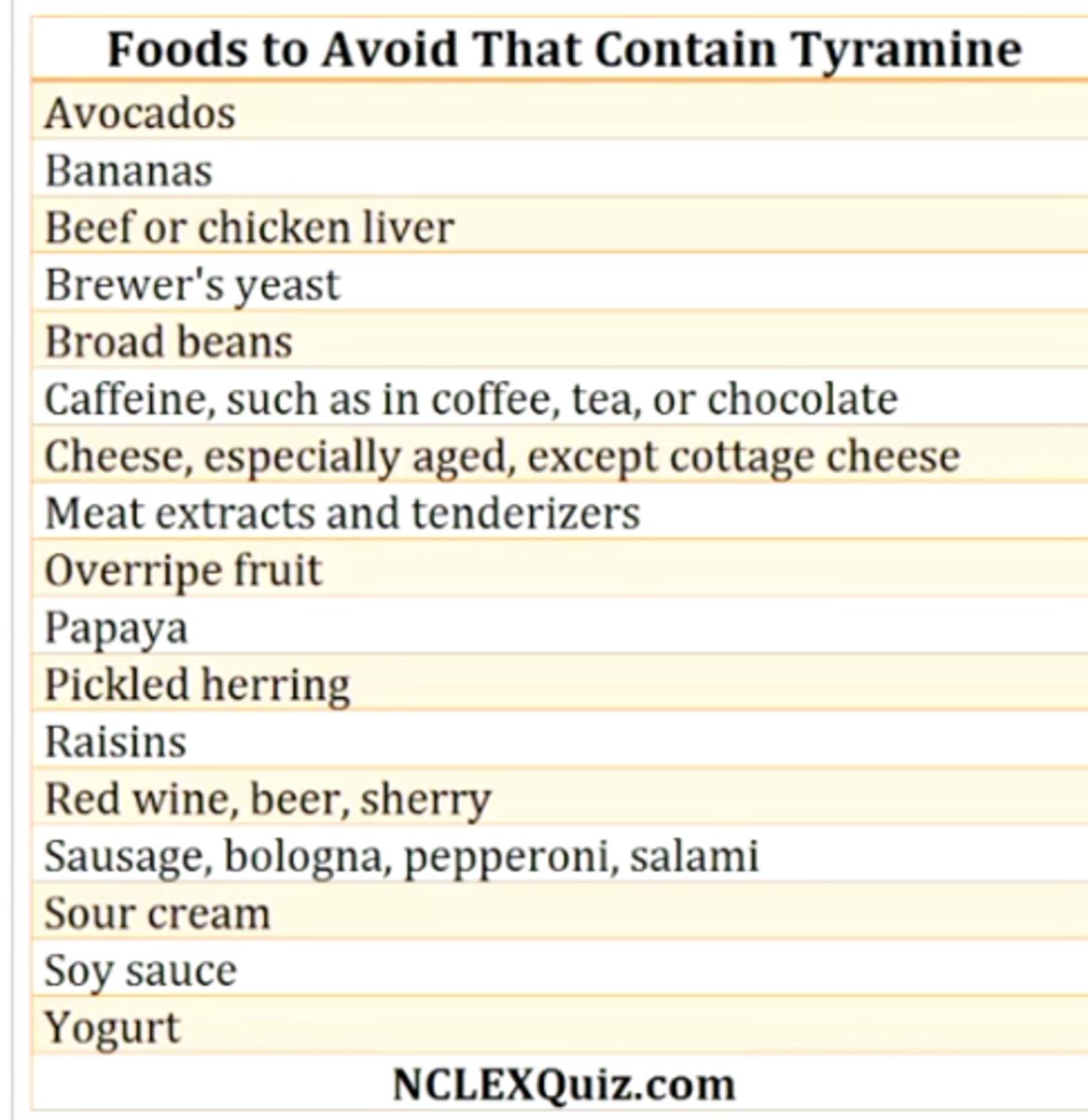
what lifestyle change do people taking monoamine oxidase A inhibitors need to make? why?
carefully watching diet to avoid foods that are high in tyramine
these foods are commonly aged, smoked, pickled, cured, fermented, overripe (avocados, bananas, cheeses, overripe fruit, red wine, certain meats)
why do you never want to combine SSRIs/SNRIs/MAOIs?
increased risk of serotonin syndrome
could include symptoms such as muscle rigidity, wet mucosa and skin, increased pupil dilation, increased bowel sounds, and increased reflexes
what 3 drugs are the "atypical" antidepressants?
1. bupropion
2. trazodone
3. mirtazapine
what is the MOA of mirtazapine?
5-HT2 antagonist/a2 antagonist/H1 antagonist
seems counterintuitive to treat depression because it reduces serotonin levels, but it's mechanism is not really clear - could be due to a2 antagonism, which increases norepinephrine
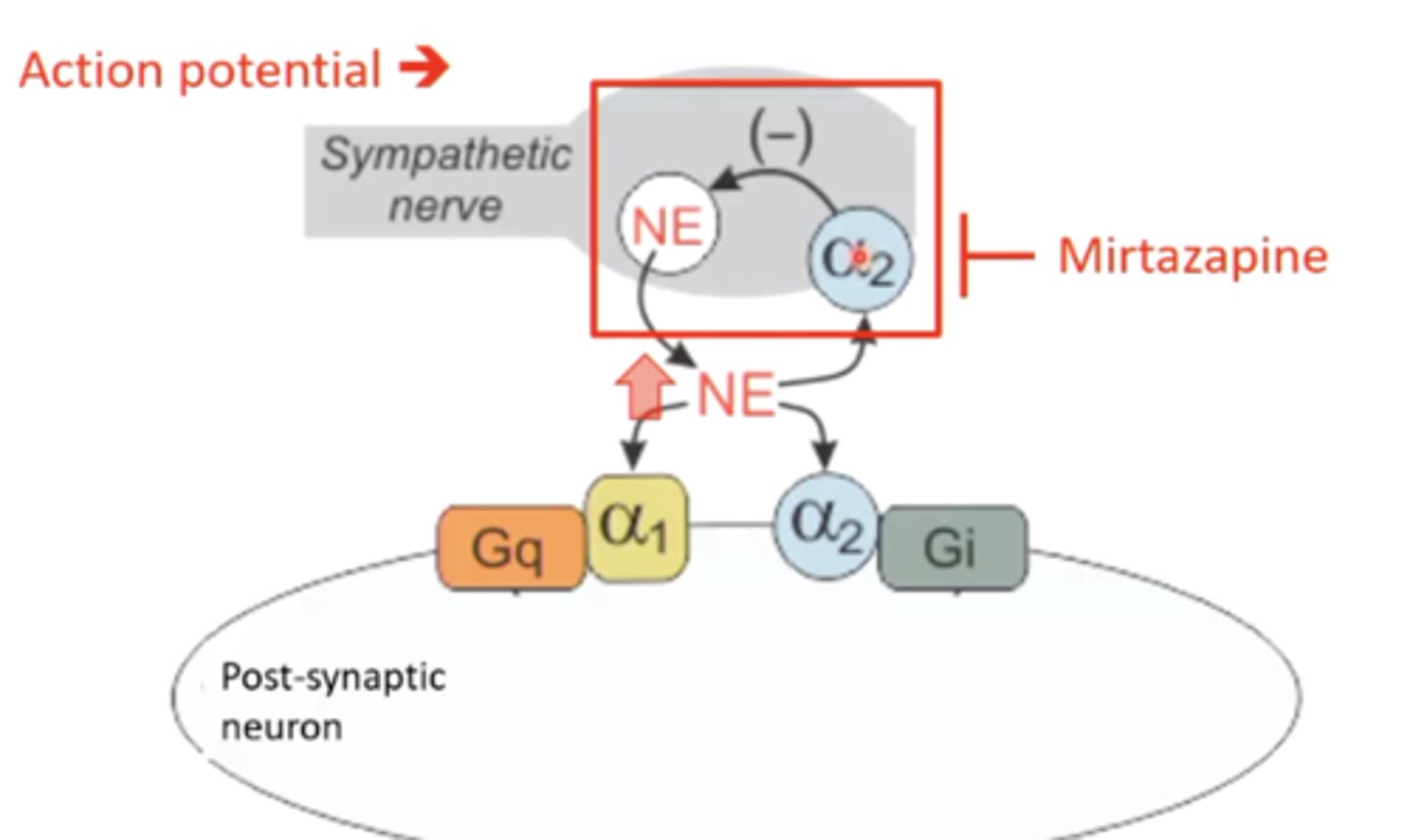
what are some toxicities associated with mirtazapine?
1. sedation due to H1 histamine receptor antagonism
2. weight gain
what is the MOA of trazodone?
5-HT2 antagonist/a1 antagonist
what is a toxicity associated with trazodone?
somnolence (excessive sleepiness)
this is why it is often prescribed for sleep
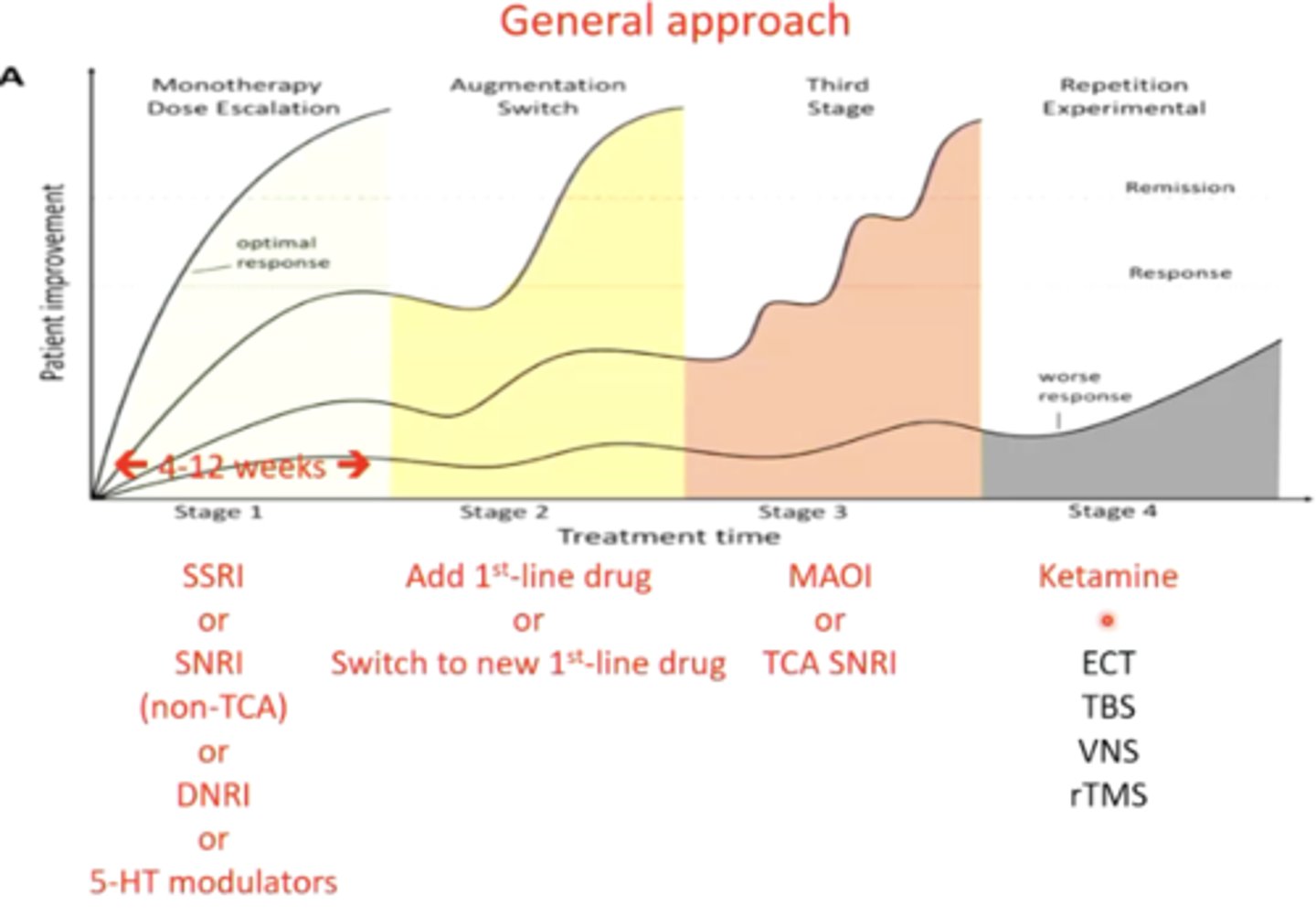
describe the general approach to prescribing anti-depressants:
first line: SSRI or SNRI (non-tricyclic)
second line: switch the SSRI or SNRI, or add another 1st line drug to make a combo
third line: MAOI or tricyclic SNRI
fourth line: ketamine
what is the MOA of ketamine?
there are many MOAs, but it is mainly a glutamate receptor antagonist
ketamine is experimentally being used to treat what condition?
refractory depression
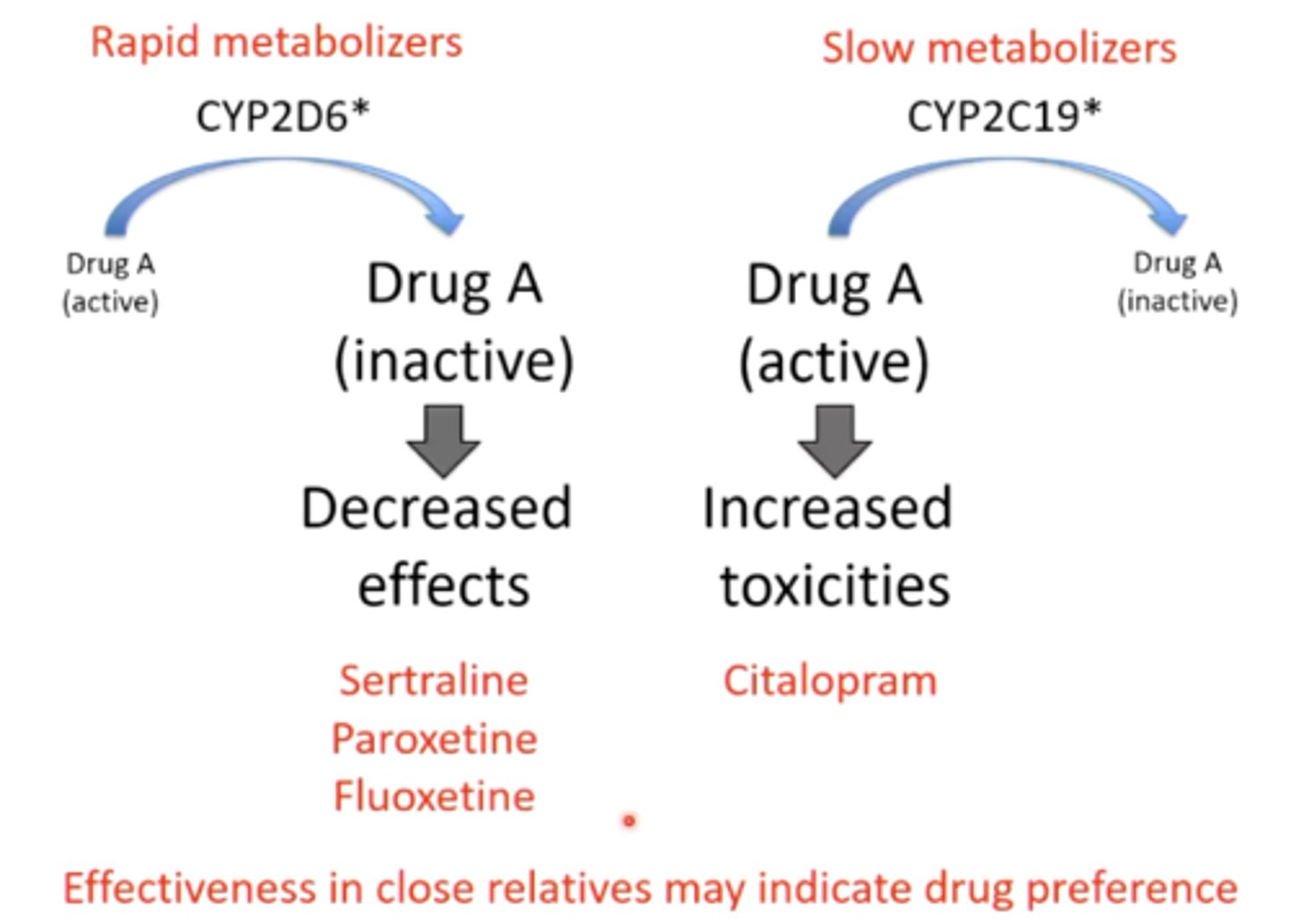
what are some methods you can use to choose between anti-depressants when first prescribing?
depends on individual efficacy (rapid metabolizers vs. slow metabolizers)
1. could ask if any close relatives are taking any effective anti-depressants and base your decision off that
2. could genotype a patient to get recommendations for which anti-depressants might work best
list the SSRIs we need to know:
1. citalopram
2. escitalopram
3. fluoxetine
4. sertraline
5. paroxetine
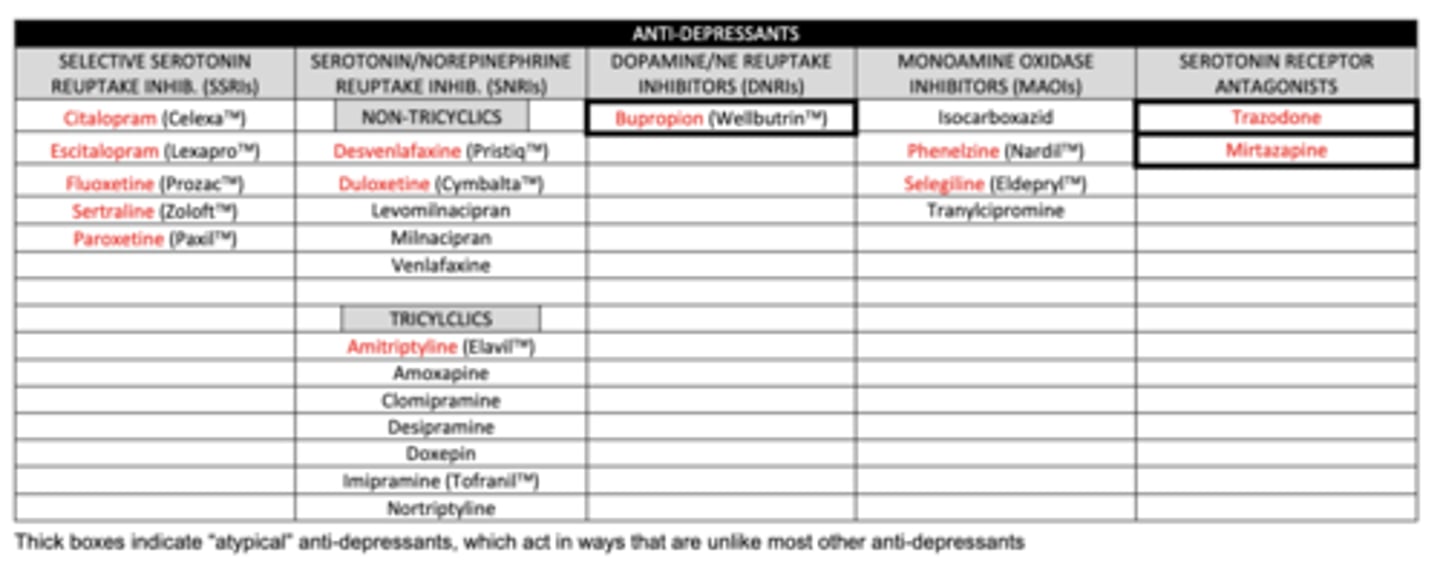
list the non-tricyclic SNRIs we need to know:
1. desvenlafaxine
2. duloxetine
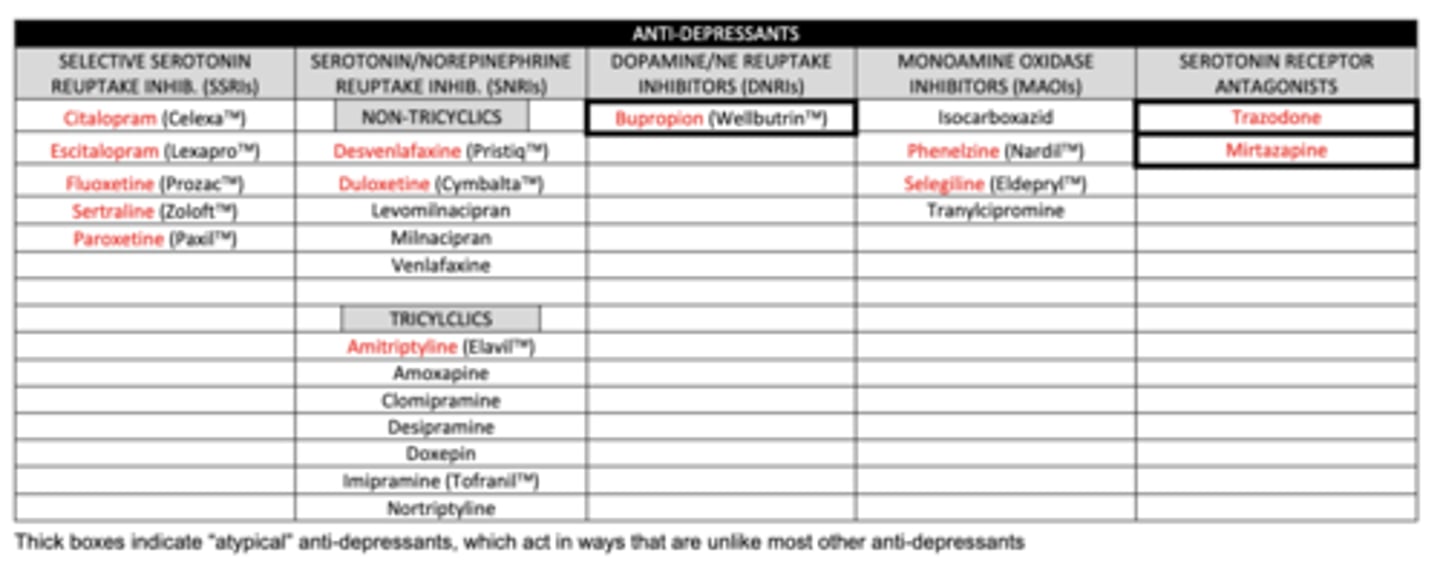
list the tricyclic SNRI we need to know:
amitriptyline
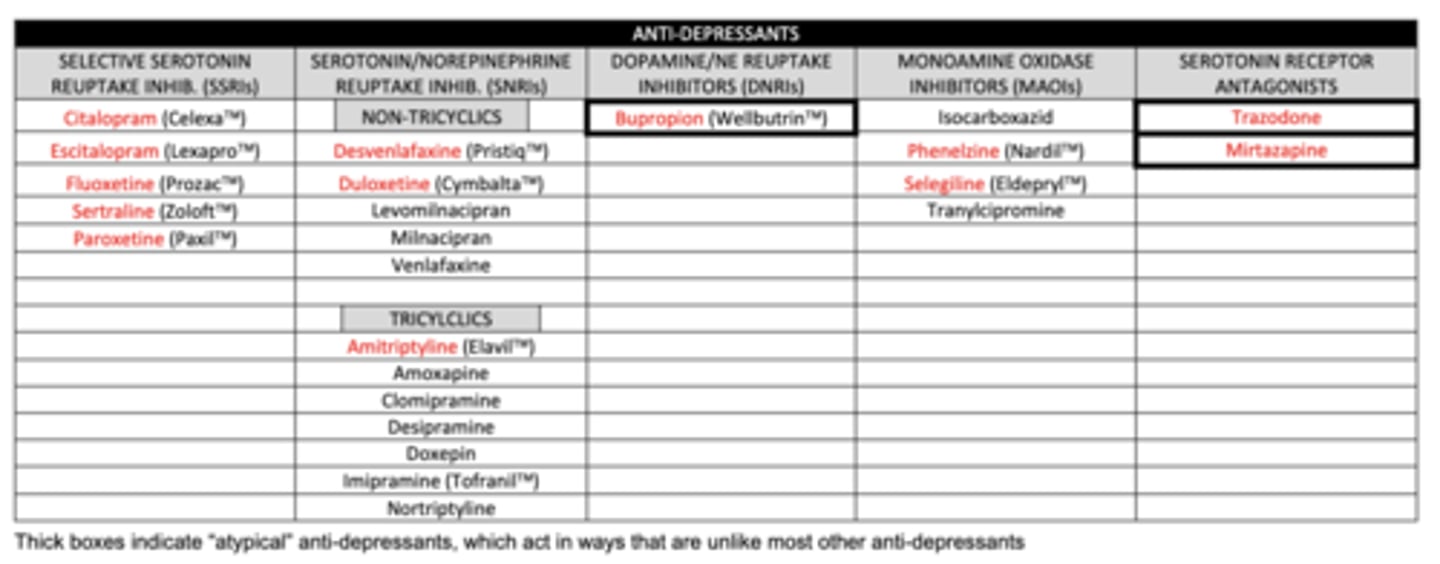
list the DNRI we need to know:
buproprion
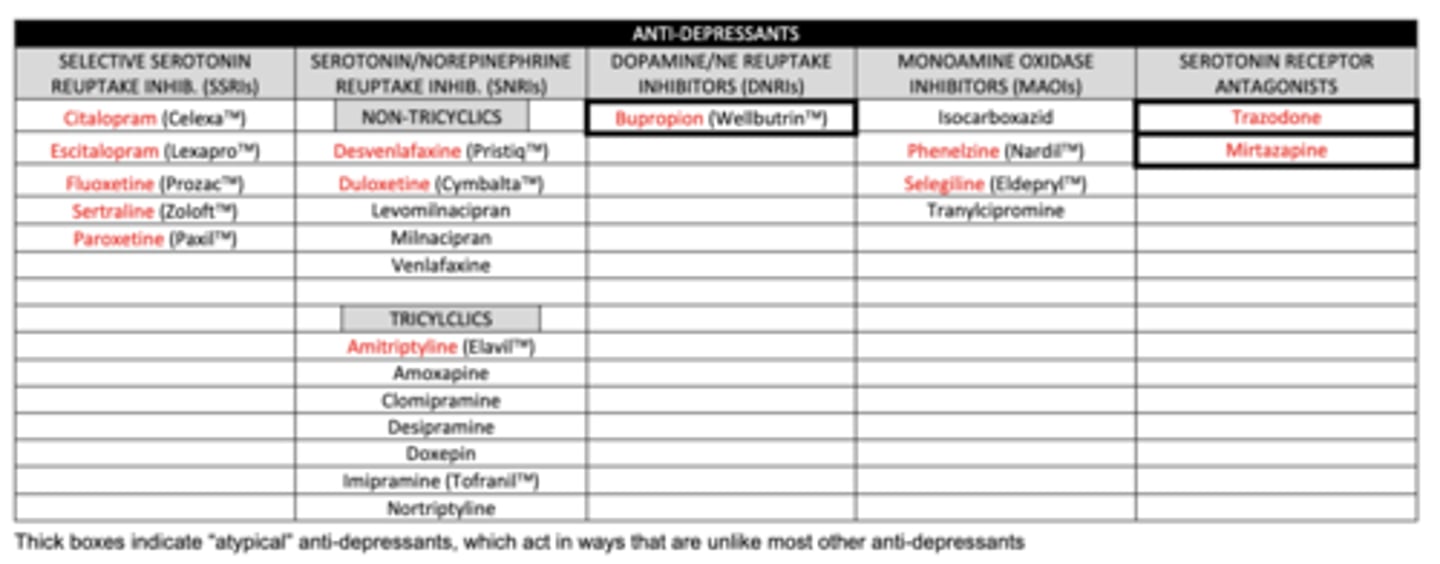
list the MAOIs we need to know:
1. phenelzine (MAO-A and MAO-B)
2. selegiline (MAO-B)
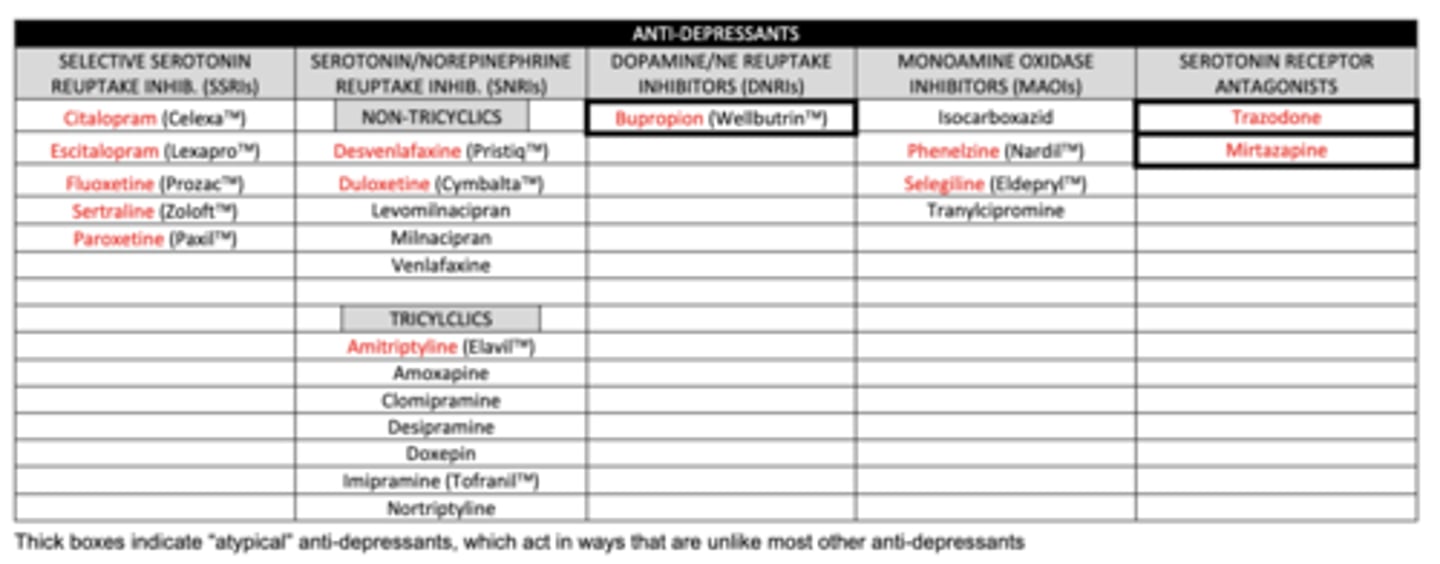
list the serotonin receptor antagonists we need to know:
1. trazadone
2. mirtazapine