Haemoglobin, Iron and Red Cell Metabolism
1/42
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
43 Terms
Haemaglobin
carries oxygen, binds and releases hydrogen ions, contributes to acid-base balance
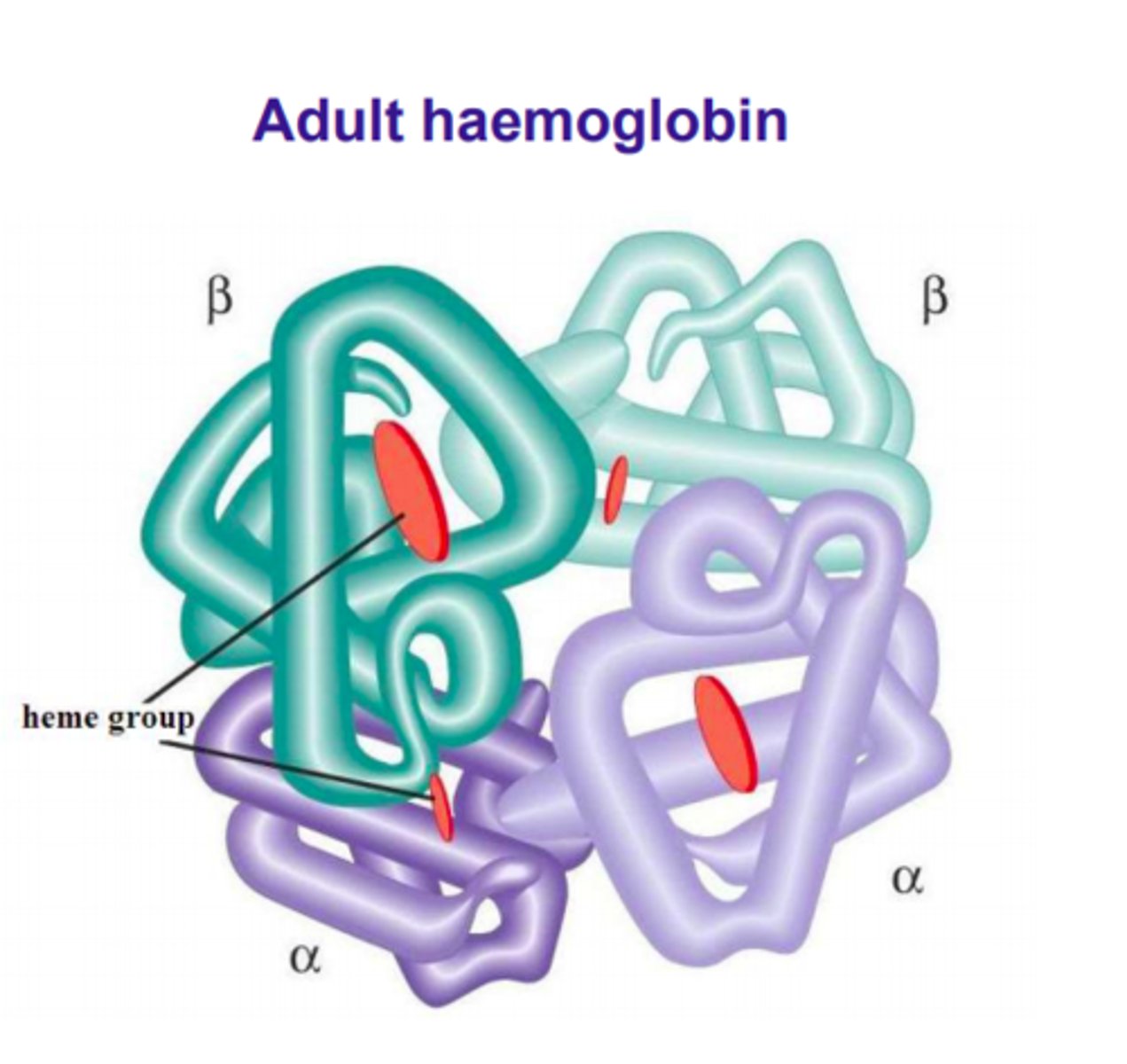
Haemaglobin structure
4 globulin chains with one heam group within an iron core. 2 alpha globulins, 2 beta globulins
Haem structure
a ring of carbon, hydrogen and nitrogen atoms (protoporphorin IX) with a central atom of divalent ferrous iron (Fe2+). ferrous iron in each haem reversible binds to one oxygen molecule.
protoporphorin IX
is a key intermediate in the heme biosynthetic pathway, serving as a precursor for heme
Haemologbin synthesis
Occurs in mitochondria and cytosol of bone marrow erythrocyte precursors. Proerythroblast to reticulocyte stage. Synthesis occurs in early stages of erythroblast so there are still ribosomes
why can mature erythrocytes not make haemaglobin
they have lost their ribosomes and mitochondria
haem synthesis
Ferric iron (Fe3+ ) is delivered to the cell by transferrin via transferrin receptors. Transferrin is recycled, Fe3+ enters the mitochondria where it is reduced to Fe2+ and united with protoporphyrin IX to form haem.
globin synthesis
Occurs in the cytosol on ribosomes. Chromosome 16 (α chains) and Chromosome 11 (non-α) control the synthesis of the globin chains.
Normally, in adults, α chains and β chains are produced in equal quantities.
2 α and 2 β chains combine. happens the same time as haem synthesis
Actions of Erythropoietin
increase number of committed progenitors which are then stimulated to proliferate, differentiate and synthesize Hb
Hb affinity for oxygen
related to the partial pressure of oxygen. The affinity of Hb for O2 is defined by the amount of O2 needed to saturate 50% of Hb (P50 value)
PO2
(PO2 ) measured in mmHg
PO2 determines the amount of oxygen needed to saturate 50% of Hb (P50 value)
high O2 tension
Hb has high affinity for O2
low O2 tension
Hb has low affinity for oxygen
Haemoglobin Oxygen Dissociation Curve
A change in pH shifts the Hb O2 saturation curve (Bohr effect).
This facilitates the ability of Hb to exchange O2 and CO2. a decreased pH in tissues shifts curve to the right
so there is lower affinity for O2
2,3-bisphosphoglycerate (2,3-BPG) concentration
molecule that stabilises the deoxygenated form of Hb
tense state
stabilises the deoxygenated form of Hb by binding between the β-globin chains, favouring O2 release;
relaxed state
Binding of O2 releases 2,3-BPG and the Hb molecules rotates into the relaxed "R" state when it is fully oxygenated
Haemaglobin function In venous blood
CO2 diffuses into RBC and combines with water. carbonic acid (H2CO3 ), then dissociates to H+ and HCO3-. H+ binds oxygenated Hb and the oxygen is released
Haemaglobin function in lungs
O2 diffuses into RBC, binds to deoxygenated Hb, release H+ of from Hb. H+ combines with HCO3 - to form carbonic acid which is converted to water and CO2
Haemoglobin Function - Nitric oxide transport
helps with relaxation of vascular smooth muscles and vasodilation
Dyshaemoglobins
Dysfunctional Hb unable to transport oxygen
Mostly acquired (drugs or toxins), some hereditary
Methemoglobin (MetHb)
Circulating Hb present with iron in oxidised Fe3+ instead of usual Fe2+ state which cannot bind to oxygen
Haemoglobin Catabolism
Is a normal process at the end of the RBC lifespan
Hb broken down to globin, iron and protoporphyrin.
Globin and iron are recycled. protoporphyrin is disposed of
iron deficiency
reduced availability of iron for haem synthesis.
thalassaemia
reduced production of globin chains (α or β) and the accumulation of the excess globins
Haemoglobinopathy
point mutations in the globin chains that lead to unstable haemoglobins.
iron distribution compartments
functional, storage and transport
functional compartment
Contains all iron that is functioning within cells
storage compartment
Iron that is not currently functioning but is available when needed
transport compartment
Iron that is in transit in the plasma (transferrin)
iron chemistry
The metabolic function of iron depends on its ability to change its valence state from reduced ferrous iron to oxidised ferric iron. In cells, ferrous iron can react with peroxide and from highly reactive oxygen molecules (including free radicals) that can damage proteins, lipids and nucleic acids
iron stores
Iron not required for erythropoiesis is stored in hepatocytes and macrophages.
Ferritin is the storage form of iron. It is mobilised to BM when required.
hepcidin
regulates iron transport to cells.
Hepcidin synthesis (in the liver ) is affected by: Hb levels Inflammation, Iron stores, Erythropoietic activity, Oxygen content
iron metabolism in other tissues
10 - 15% of iron is in myoglobin and cytochromes.
Myoglobin is the oxygen carrying molecule for muscles and resembles haemoglobin
full blood count assessment
RBCC, HGB, MCV and RDW. also checks size, colour and shape
serum iron studies
indicator of available transport iro
transferrin studies
indicator of available transport iron and transferrin level
ferritin
indicator of available storage iron
loss of deformability
decreased mean cell haemoglobin concentration means deformability compromised shortened red cell life span
deformability
process of RBCs to stretch undamaged up to 2.5 times their resting diameter as they pass through narrow capillaries and splenic pores
RBC energy generation
produce ATP (adenosine triphosphate) through anaerobic glycolysis. ATP also required to slow down the oxidation of proteins and iron by environmental peroxides and superoxide anions to maintain haemoglobin function and membrane integrity
Embden Meyerhof pathway
RBC lack mitochondria so rely on anaerobic glycolysis for energy generation.
Metabolism of glucose to pyruvate.
2 ATPs produced per glucose molecule. energy is used to maintain volume shape and flexibilty. NADH also produced, necessary for activation of non-functional MetHb
Hexose Monophosphate pathway
G6PD produces NAD and NADH (antioxidants prevents iron from getting oxidized). G6PD is pivotal as it is the only means for the RBC to generate NADPH which reduces glutathione.
Reduced glutathione reduces peroxide to water