Topic 6: Ocean Acidification
1/23
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
24 Terms
Pteropods
Free-swimming sea snails with wing-like flaps
found in upper 10 m of all oceans
Some shelled, some not shelled
Important source of food – ‘potato chips of the sea’ for mackerel, salmon, herring, whales
Pteropod experiment
Shells placed in sea water with pH and carbonate levels projected for the year 2100
Pteropod experiment results
Damaged shells (ridges, cloudy, weak spots) due to ocean acidification
Ocean acidification
Consequence of excess carbon dioxide in the atmosphere resulting in excess CO2 in seawater (and therefore decreased pH)
Time for ocean system to buffer
1,000 to 100,000 years
pH scale
Specifies the acidity or basicity of an aqueous solution
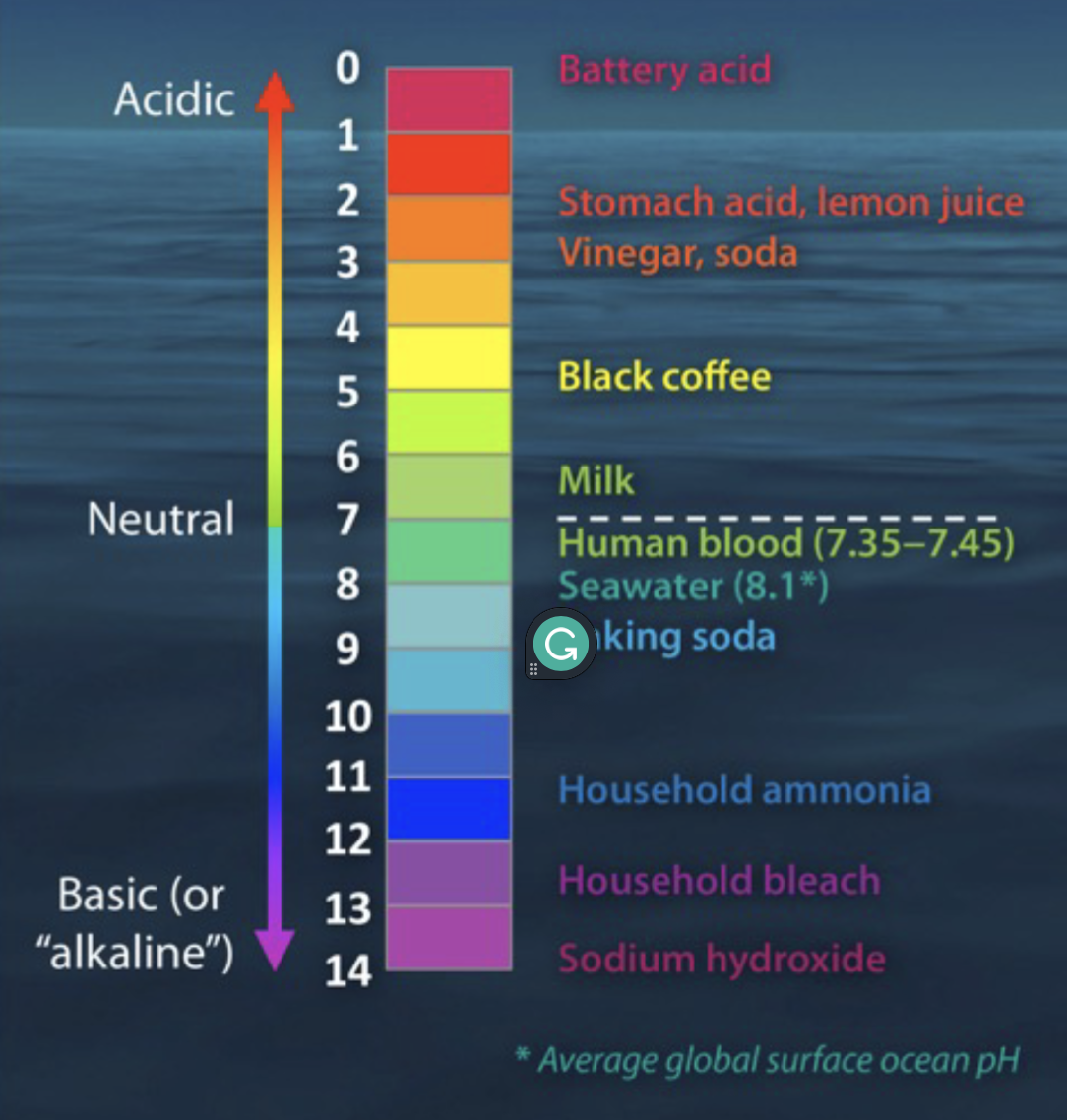
Acidity
Concentration of H+ in solution; with increased concentration of H+, pH decreases
Buffering
Minimize pH changes (doesn’t work in ocean right now because of how fast pH is dropping)
Two ocean buffering reactions
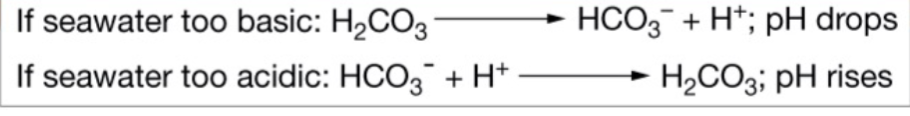
Ocean acidification reaction
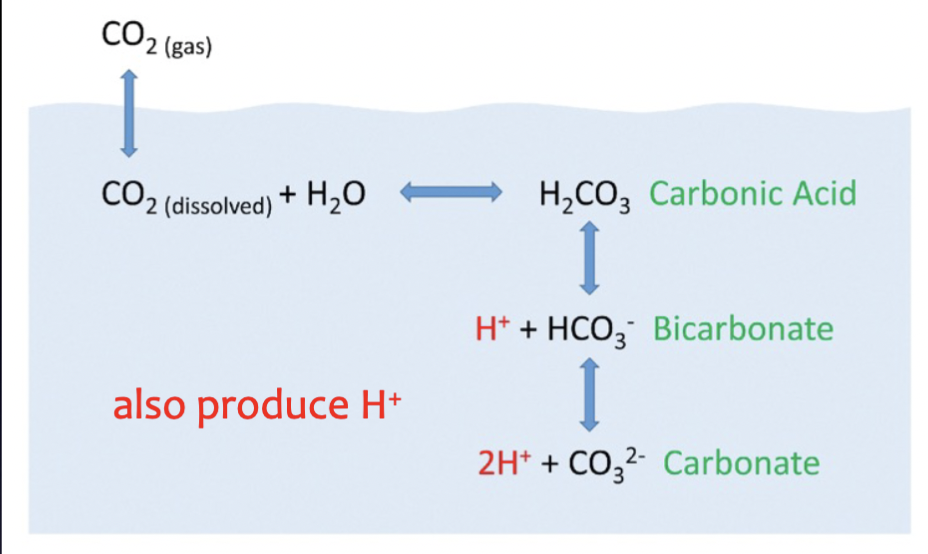
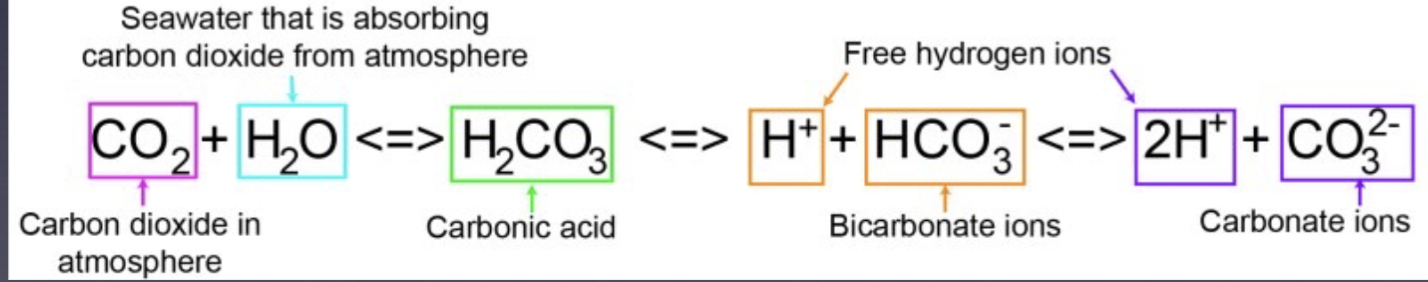
3 types of carbon created when CO2 enters ocean (in order)
carbonic acid
bicarbonate ion
carbonate ion
Dissolved inorganic carbon (DIC)
Total amount of carbonic acid, bicarbonate ion, and carbonate ion in the ocean
Step 1: ocean acidification
Atmospheric CO2 combines with seawater to form carbonic acid, then form bicarbonate ions and free H+
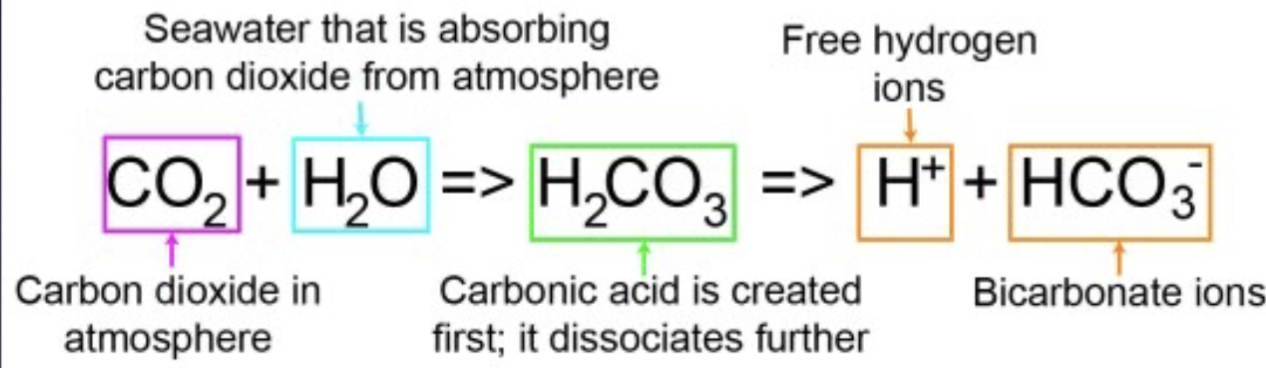
Step 2: ocean acidification
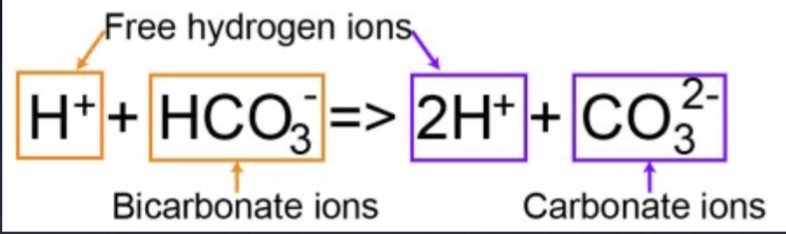
Bicarbonate ions dissociate
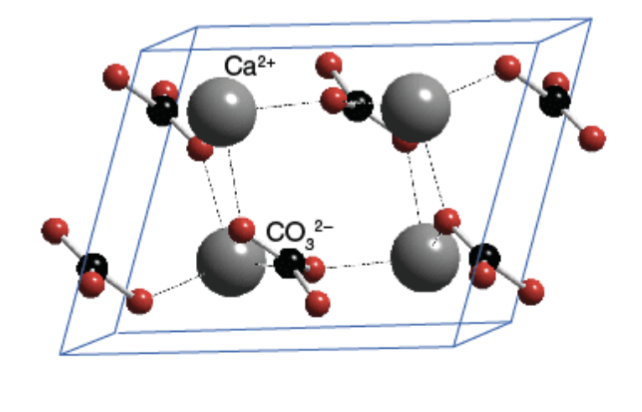
What are calcite and aragonite
Polymorphs of CaCO3
4 Characteristics of calcite
Trigonal crystal system
Stable
Varying amounts of Mg²+
Plankton, sponges, brachiopods,
echinoderms, bivalves (parts of
shell
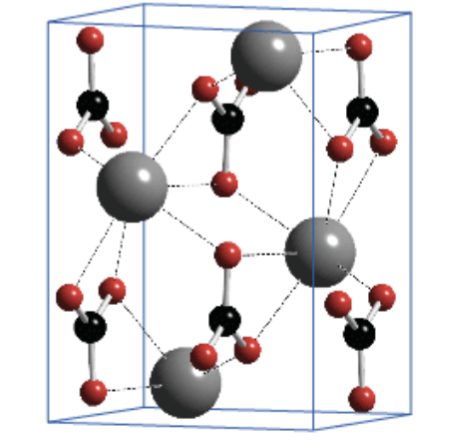
5 Characteristics of aragonite
Orthorhombic crystal system
Strong at higher P
Metastable state
Greater solubility
Corals, pteropods, most molluscs
Type of ocean we live in today (calcite vs aragonite?)
Aragonite sea
Calcite vs aragonite seas
Shift over geologic time, means that different organisms are reef-builders
3 Causes of shifting calcite vs aragonite seas
Climate cycles
Submarine volcanism
Seafloor spreading rates
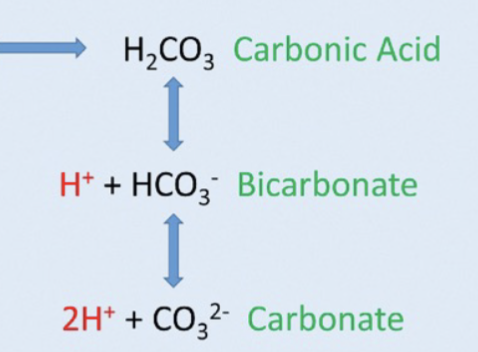
Step 3: ocean acidification
Steps 1 and 2, moving right or left
3 Climate effects of ocean acidification
Decrease in the pH (increasing acidity) of the Earth's oceans (caused by the uptake of carbon dioxide (CO2) from the atmosphere)
Difficult for organisms (calcifiers) to create hard parts (less carbonate available + requires more energy)
Decrease in the ocean’s capacity to absorb CO2
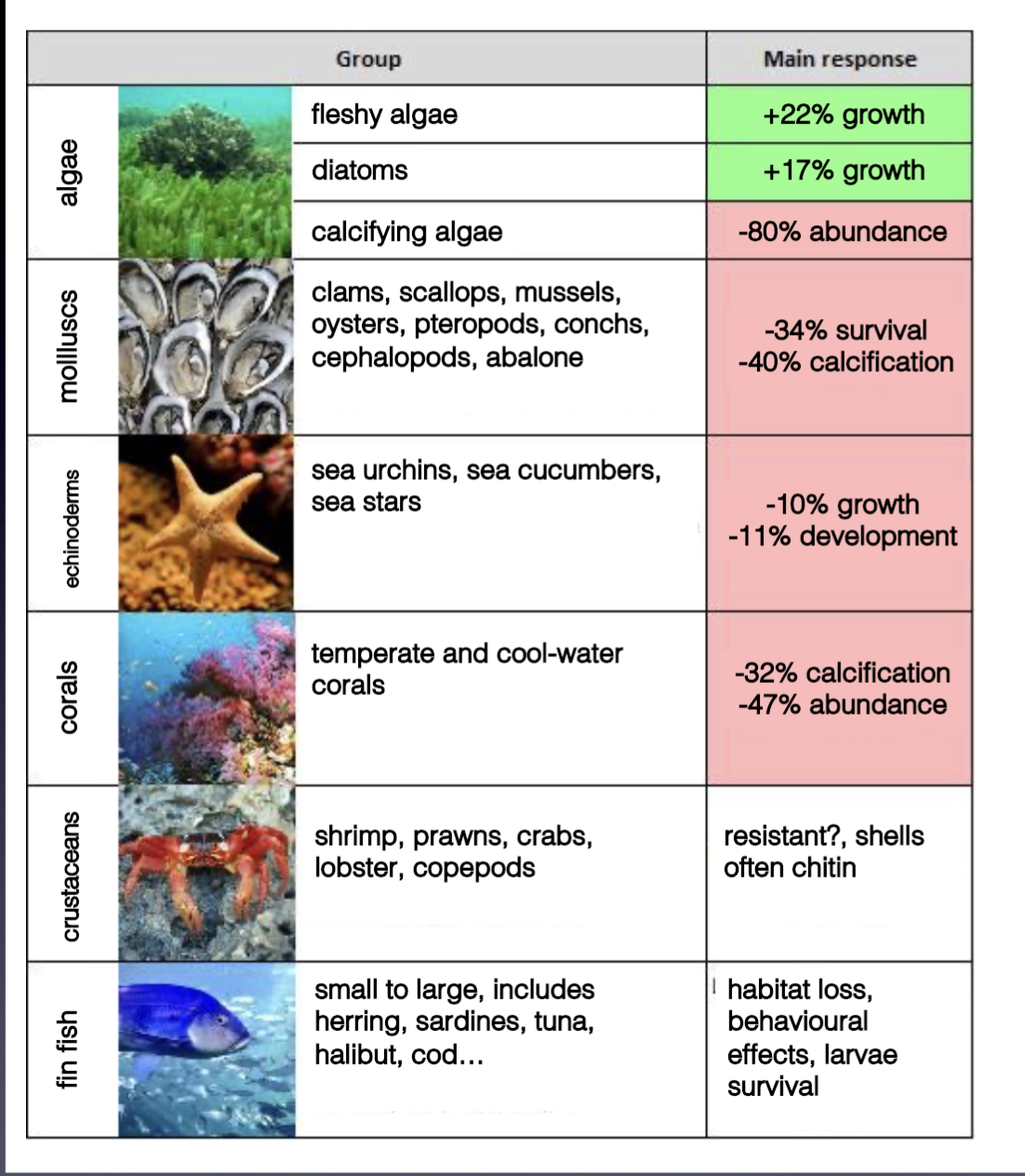
Effects of ocean acidification on specific organisms
For calcifiers: smaller shell sizes, more ‘fragile’ shells, dissolving shells, low reproductive rates in oysters
Weaker byssal threads in mussels (cannot stay attached to things as easily)
4 Effects of ocean acidification on humans + animals
Loss of biodiversity
Affects food security
Money loss (aquaculture, tourism)
Loss of coastal protection