Honors Chemistry Final - Sophomore Year
1/216
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
217 Terms
UNIT 5
UNIT 5
Covalent bonding is…
sharing electrons between 2 or more nonmetals
ELECTRONEGATIVITY DIFFERENCE VS BOND TYPE
ELECTRONEGATIVITY DIFFERENCE VS BOND TYPE
Electronegativity difference of an ionic bond
>1.8
Electronegativity difference of a polar covalent
between 0.4-1.8
Electronegativity difference of a nonpolar covalent
<0.4
Covalent bonding =
molecular bonding
What does this mean?
Covalent Bonding means…
Bond = Covalent
Molecular Bonding means…
Particle formed = molecule
In a Covalent Bond…
electrons are shared so that atoms achieve an octet
What are the two types of covalent bond?
Polar and Nonpolar
Polar Covalent bond
electrons are NOT shared equally
Nonpolar covalent bond
electrons are shared equally
Lewis Dot Structures
represent valence electrons using dots
Example of a Lewis dot structures
Carbon - 4 valence electrons
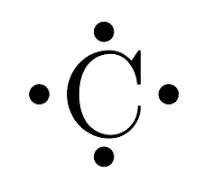
What is a lone pair?
an unshared pair of electrons/dots
what are bonding electrons?
the electrons that are not paired
NAMING COVALENT COMPOUNDS
NAMING COVALENT COMPOUNDS
step 1 for naming covalent compounds
Name the first element, add prefix to indicate the number of atoms
DON’T ADD “MONO” IF THERE IS ONLY ONE OF THE FIRST ELEMENT
Step 2 for naming covalent compounds
Name the second element, change the ending to “ide” Add prefixes to indicate the number of atoms
What Are The Prefixes?
1 mono-
2 di-
3 tri-
4 Tetra-
5 Penta-
6 Hexa-
7 Hepta-
8 Octa-
9 Nona-
10 Deca-
WHEN YOU ADD A PREFIX TO OXYGEN…
DROP THE “a” OR THE “o” AT THE END OF THE PREFIX
NAMING COVALENT COMPOUND PRACTICE
NAMING COVALENT COMPOUND PRACTICE
N2O3
Cl2O7
BCl3
CF4
Dinitrogen Trioxide
Dichlorine Heptoxide
Boron Trichloride
Carbon Tetrafluoride
COVALENT COMPOUND FORMULA PRACTICE
COVALENT COMPOUND FORMULA PRACTICE
Dinitrogen Tetrahydride
Trisilicon Tetranitride
Dichlorine Heptoxide
Phosphorus Pentabromide
N2H4
Si3N4
Cl2O7
PBr5
DRAW LEWIS STRUCTURES FOR MOLECULES
DRAW LEWIS STRUCTURES FOR MOLECULES
step 1
Count the total number of valence electrons from all atoms in the molecule
step 2
Choose your central atom
(This should be the LEAST electronegative atom in the molecule)
Step 2 Tips
Carbon is ALWAYS the central atom
Hydrogen is NEVER the central atom
Halogen are USUALLY NOT the middle
step 3
Arrange the remaining atoms around the central atom and attach using single bonds
step 4
Add lone pairs to any atoms to achieve octets
What are two elements that DO NOT need an octet and how many electrons do they actually need?
Hydrogen (needs only 2 electrons)
Boron (only needs 6 electrons)
step 5
Adjust using double or triple bonds as needed for the total electrons or octet to match
Examples of drawing structures
Examples of drawing structures
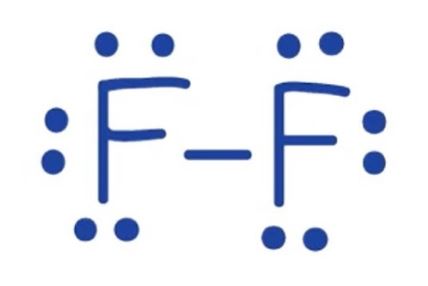
F2
= 14 total valence electrons
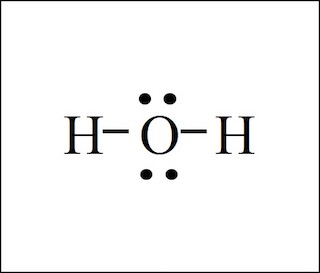
H2O
2(1) + 6 = 8 total valence electrons
(Hydrogen is never in middle)
(There are 2 shared pairs and 2 unshared pairs)
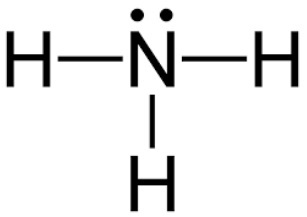
NH3
5 + 3(1) = 8 valence electrons
CBr4
4 + 7(4) = 32 valence electrons
(Carbon is always middle)
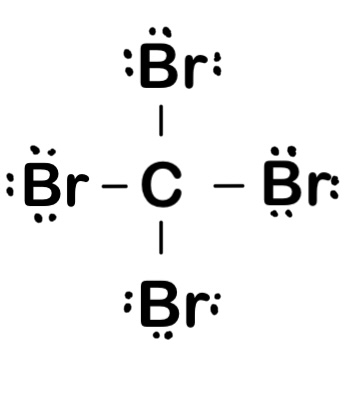
PCl3
5 + 7(3) = 26 valence electrons
How many electrons does each bond count for?
2
What is an octet?
a full set of 8 valence electrons
everyone/every element wants this
The noble gases have this
How do we know when to use double or triple bonds?
How do we know when to use double or triple bonds?
examples
examples
O2
12 electrons
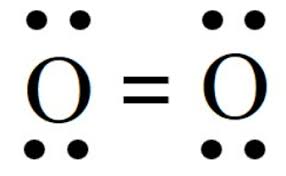
N2
10 electrons
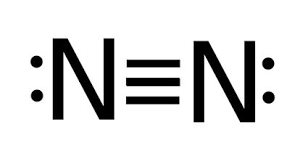
CO2
16 electrons
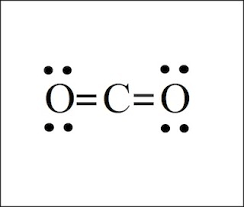
SiS2
16 electrons
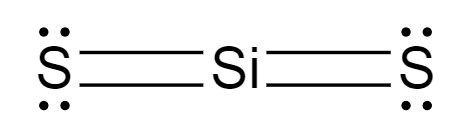
How Do We Draw Structures For Polyatomic Ions?
It is the same as drawing lewis structures for covalent compounds but…
Add electrons to account for the charge
Add brackets and the charge when structure is complete
examples
examples
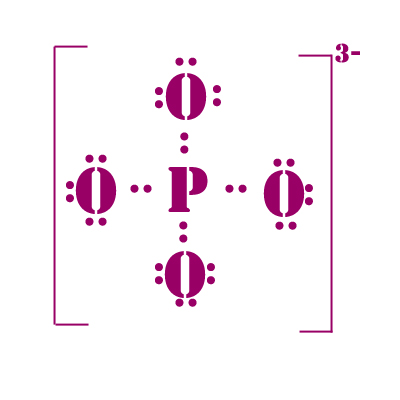
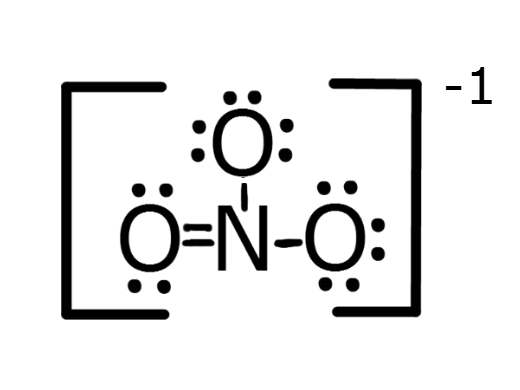
PO4 3-
5 + 4(6) + 3 = 32 electrons
ClO3-
7 + 3(6) + 1 = 26 electrons
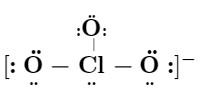
NO3-
5 + 3(6) + 1 = 24 electrons
VSEPR THEORY
VSEPR THEORY
vsepr stands for…
Valence Shell Electron Pair Repulsion
what is vsepr theory?
when molecules form the electron pairs in bonds and lone pairs spread as far apart from one another as possible
What do we do for it?
focus on central atom
count the e- domains surrounding that atom
What are electron domains?
bonds (single, double, or triple) or lone pairs (around the central atom)
examples
examples
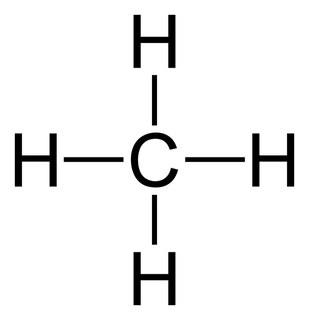
CH4
8 electrons
4 electrons domains (all are bonds)
molecular geometry: tetrahedral
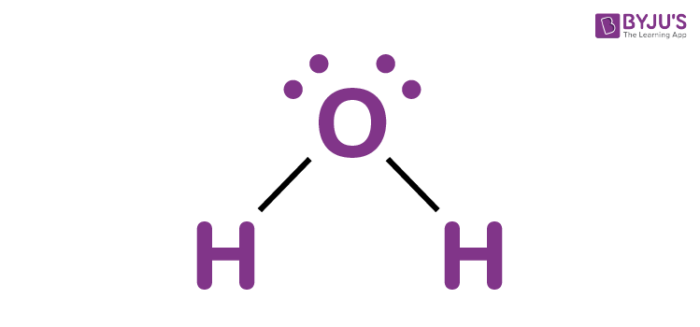
H2O
8 electrons
4 electron domains (2 lone pairs, 2 bonds)
molecular geometry: tetrahedral bent
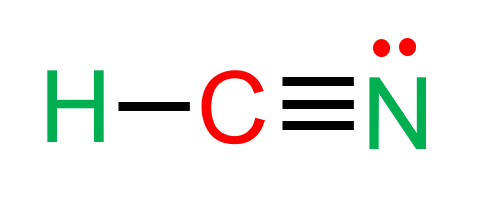
HCN
10 electrons
electron domains: 2 (both bonds)
molecular geometry: linear
BF3
3 +3(7) = 24 electrons
electron domains: 3 (all bonds)
molecular geometry: trigonal planar
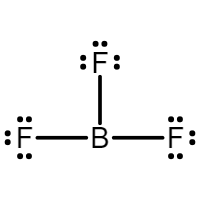
EXPANDED OCTET
EXPANDED OCTET
what is an expanded octet?
some electrons in the 3rd period (below 3A-7A) or lower can expand their octet (only when there is no other option)
EXPANDED OCTET IS THE…
EXCEPTION NOT THE RULE
examples
examples
SF6
6 + 6(7) = 48 electrons
6 bonds, 0 lone pairs
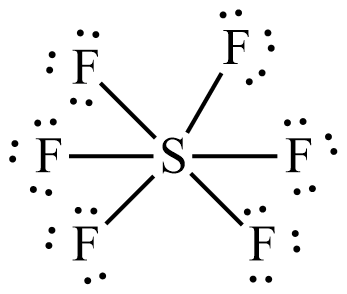
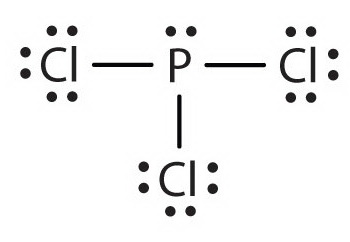
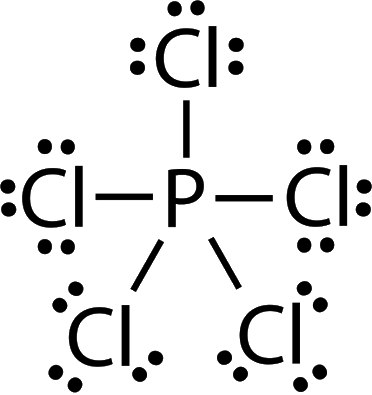
PCl5
5 + 5(7) = 40 electrons
5 bonds, 0 lone pairs
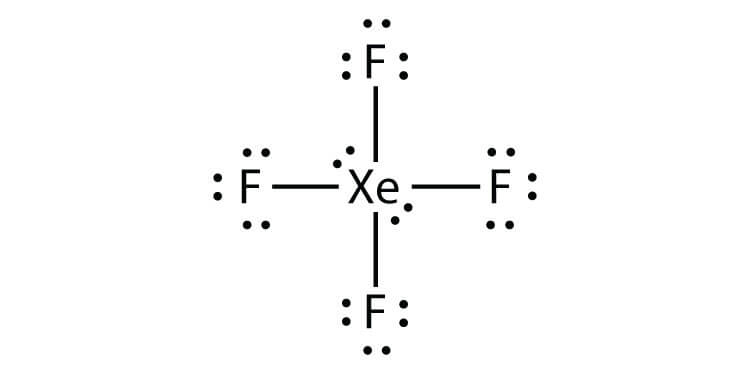
XeF4
8 +4(7) = 36 electrons
4 bonds, 2 lone pairs
IF EXTRA ELECTRONS…
ADD TO CENTRAL ATOM
What is an intermolecular force?
attractions that exist between molecules
key words: “between molecules”
what is the imf responsible for?
properties such as:
melting/boiling point
surface tension
solubility
WHAT CAUSES POLARITY IN MOLECULES?
WHAT CAUSES POLARITY IN MOLECULES?
what causes polarity in molecules?
an unequal distribution of electron density, due to a difference in electronegativity values
Nonpolar
No central atom, no lone pair, bonded to all of the same atom it’s nonpolar
ex: H2, Cl2, CCl4, BF3
Polar
Where you see the dash, that’s the partially positive side
Anytime the central atom is bonded to atoms of different elements, the molecule of polar
anytime there is a lone pair(s) on central atom, the molecule is polar
ex: HCl, CCl3H, HClH3
THREE MAIN CATEGORIES OF IMFS
THREE MAIN CATEGORIES OF IMFS
London Dispersion Forces (LDF)
all molecules have LDF between them
the weakest of the three types
the only forces that exist between nonpolar molecules
LDFs are caused by…
the movement of electrons within the electron cloud of molecules
LDFs cause…
temporary dipoles within those molecules
extra info on LDFs…
also known as van Der Waals forces
Instantaneous attraction (why it’s so weak)
more electrons in the electron cloud means stronger LDFs
Dipole-Dipole Attraction
forces between all polar molecules
D-D forces are stronger than LDFs but weaker than Hydrogen bonding
Dipoles in polar molecules are not temporary
ex: PCl3S
DIPOLES POINT TOWARD…
THE MOST ELECTRONEGATIVE ATOM
Hydrogen Bonding
specific type of Dipole-Dipole
molecules that have hydrogen bonded to Fluorine, Oxygen, or Nitrogen can have hydrogen bond with one another
the strongest IMF
a very strong coulombic attraction
Hydrogen bonding with F,O, or N rule
Must see H-F, H-O, or H-N
F,O,N are the most electronegative atoms on the PT
H = only reactive element with only one energy level
has a largely unshielded nucleus
Molecular Geometry
Molecular Geometry
2 electron dense areas (*know this)
linear (no lone pairs)
3 electron dense areas (*know this)
trigonal planar (no lone pairs)
bent (1 lone pair)
4 electron dense areas (*know this)
tetrahedral (no lone pairs)
trigonal pyramidal (1 lone pair)
bent (2 lone pairs)
5 electron dense areas
trigonal bipyramidal (no lone pairs)
sawhorse (1 lone pair)
T-shaped (2 lone pairs)
Linear (3 lone pairs)
6 electron dense areas
Octahedral (no lone pairs)
Square pyramidal (1 lone pair)
Square Planar (2 lone pairs)
T-shaped (3 lone pairs)
linear (4 lone pairs)
UNIT 6
UNIT 6
Signs of chemical reactions
temperature change: a gain or loss of energy in the system
change in color
change in odor
producing gas (bubbles)
production of a precipitate
what is a precipitate?
a solid formed by 2 liquids mixing
what do these signs mean?
something new is produced
What are chemical reactions represented by?
Chemical Equations
A + B → C + D
reactants → products
Symbols used in chemical reactions
(s) solid
(g) gas
(l) liquid
(aq) aqueous
→← reversible reaction
triangle over arrow heat is added to reaction
pt over arrow or chemical symbols above an arrow is a catalyst
What is a substance that dissolved in water called?
a solution
What does a catalyst do?
speeds up the reaction but does not take part in the reaction
HOW TO BALANCE CHEMICAL EQUATIONS
HOW TO BALANCE CHEMICAL EQUATIONS