inflammation (L16)
1/39
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
40 Terms
define acute inflammation
the process of inflammation through which our bodies tissues initially respond to infection or injury
local clinical features of acute inflammation
redness - caused by vasodilation - histamine and prostaglandin
swelling - caused by inflammatory exudate from increased vascular permeability - histamine and leukotrienes
heat - caused by vasodilation - histamine and prostaglandin
pain - caused by tissue damage - prostaglandin and bradykinin
loss of function - caused by tissue damage - ROS, NO, lysosomal enzymes
what are three patterns of acute inflammation
purulent (supparutive) inflammation
serous inflammation
fibronous inflammation
purulent inflammation
characterised by the production of pus (an inflammatory exudate rich in neutrophils and fluid and liquified debris of necrotic cells)
caused by pyogenic organisms that cause tissue necrosis and liquefaction
abscess is a localised collection of pus
serous inflammation
characterised by a fluid-rich, cell poor exudate
occurs in peritoneum, pleura, pericardium
other examples: skin blister or a runny nose
fibrinous inflammation
characterised by a fibrinogen-rich exudate and fibrin deposition
seen in pericardium, peritoneum
usually a part of coagulation cascade (fibrin to fibrinogen)
steps in acute inflammatory response
recognition - pattern recognition receptors and vascular changes
recruitment - of leukocytes
removal of the agent - killing and degradation - ROS/NO and phagocytosis
resolution
two types of sentinel cells important in acute inflammation (recognition)
macrophages and mast cells
macrophages
monocytes which differentiate in the tissue
long lived in tissue
phagocytic
produce pro-inflammatory cytokines in response to damage or pathogens
mast cells
tissue resident cells that can be long lived
contain numerous granules
release chemical mediators such as histamine, leukotrienes and prostaglandin
released chemicals mediate vaascular changes, pain
mast cells can survive degranulation
pattern recognition receptors - what
on macrophages and mast cells (sentinel cells in the tissues)
detect DAMPs (damage associated molecular patterns) and PAMPs (pathogen associated molecular patterns)
what does detection of DAMPs cause release of
proinflammatory cytokines including
TNF alpha
IL-1beta
IL-6
what does detection of PAMPs cause release of
chemical mediators including:
histamine
prostaglandin
leukotrienes
acute inflammation can be measured by
increased temperature
increased acute phase proteins in serum (e.g. C-reactive protein, fibrinogen - measured by erythrocyte sedimentation rate)
increased neutrophils in blood
macrophages and mast cells in tissues produce
prostaglandin - vasodilation
leukotrienes - vascular permeability
histamine - vasodilation and vascular permeability
vascular changes - WBCs
normally occassional resident lymphocytes or macrophage
inflamed = increased blood flow → leakage of plasma proteins = odema → neutrophil emigration
increased vascular permeability
inflammation causes dilation (so more blood) and retraction of endothelial cells
so fluids and proteins can pass
neutrophils
large granular cells with a multi-lobed nucleus
recruited into tissues in response to inflammatory cytokines (e.g. IL-1beta and TNF alpha)
phagocytic
make up 40-70% of white blood cells in peripheral blood
short-lived half-life 4-10 hours in circulation, 1-2 days in tissue
monocytes
large cells with kidney shaped nucleus
phagocytic
produce pro-inflammatory cytokines (IL-1beta, TNF alpha)
make up 2-10% of peripheral blood mononuclear cells
circulation time 20-40 hours in blood
diapedesis
the process of the cells in the blood moving through the endothelial wall of the vessel to the tissues
movement of leukocytes from the blood into the affected tissue
1 cytokines produced by macrophages in tissues
2 vessels become leaky and sticky
3 margination - slower blood flow and increased viscocity (congestion) throws leukocytes at the vessel wall
4 rolling - leukocytes attracted by chemokines/cytokines
5 adhesion - leukocytes tick to vessels
6 diapedesis - leukocytes migrate through vessels
7 chemotaxis - leukocytes attracted by chemokines esp IL-8
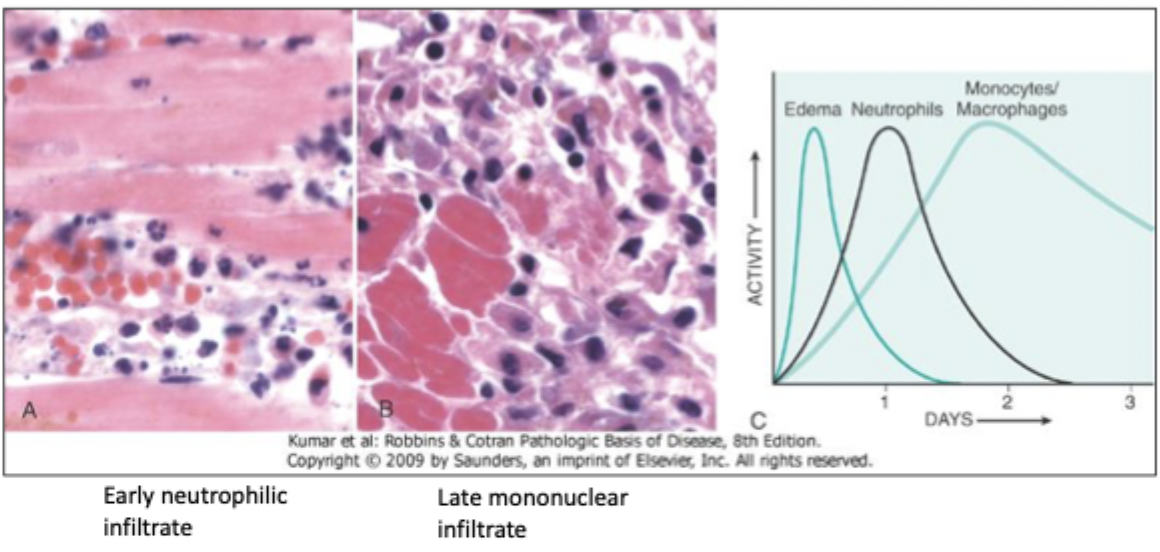
kinetics of acute inflammation
first is edema, then neutrophils, and then monocytes/macrophages
removal - what
destruction of microbes and other offenders through phagocytosis or intracellular killing
macrophages and neutrophils - how removal
ROS, NO, lysosomal enzymes → microbial actions, phagocytosis and killing of bacterial and fungi
the complement system - what
soluble proteins and their membrane receptors
host defense against microbes, removal of particulate substances (damages cells, microbes, immune complexes), modulation of immune response
leads to inflammation, lysis, and opsonisation
the complement system - types
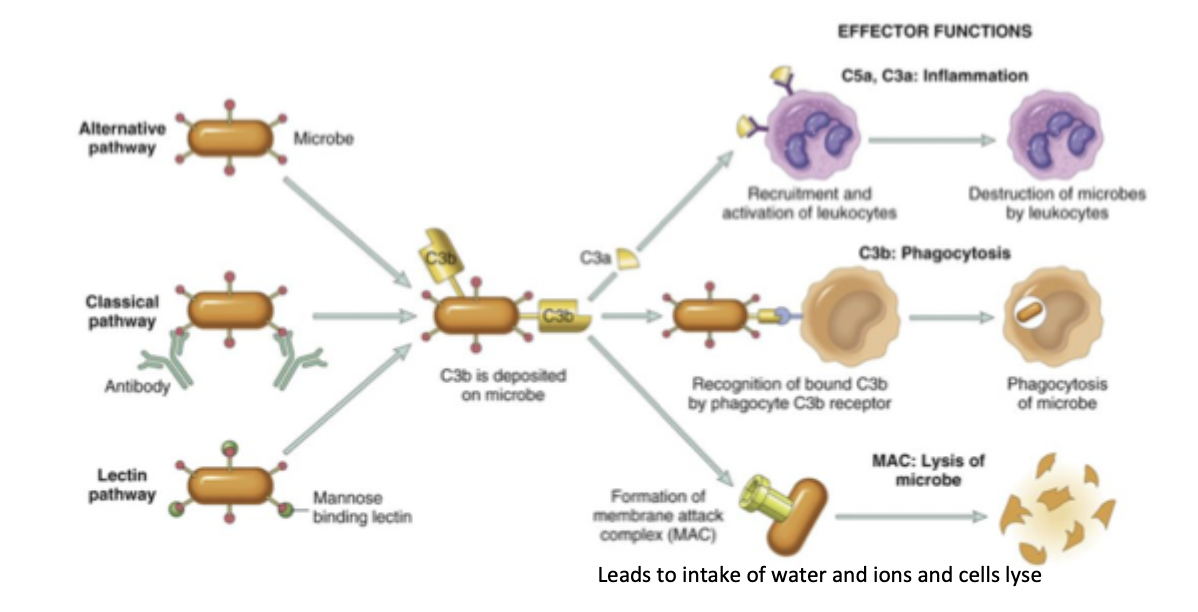
opsonisation
complement components bind to microbes
agglutination
microbes are stuck together using complement
phagocytic cells have complement receptors; opsonisation and agglutination enhance phagocytosis, increasing clearance of pathogens
lysis
formation of membrane attack complex, perforating the cell wall and release of contents
kills the target organism
inflammation
binding of complement to leukocytes increases adhesion to endothelium
increased chemotaxis (cell migration); macrophages and neutrophils are recruited
osteomyelitis
inflammation of the bone
neutrophils to the site
spread to the periosteum
subperiosteal abcess may form and impair blood supply causing necrosis
dead bone can be released into the sinus tract
resolution
inflammation decline when offending agents removed
chemical mediators have a short half lide and are destroyed by degradative enzymes
neutrophils have a short lifespan in the blood, die and are themselves phagocytosed
activated macrophages secrete IL-10 which downregulates macrophage responses
acute inflammation outcomes
acute inflammation → healing process → resolution or scarring or unhealed wound (ulcer)
OR
acute inflammation → chronic inflammation → unhealed wound (ulcer)
what cause chronic inflammation
prolonged response to persistent stimuli
persistent infections, e.g. mycobacteria, fungi, parasites
immune mediated diseases - asthma, IBD, RA, MS
prolonged exposure to toxic agents (e.g. asbestos, silica)
features of chronic inflammation
inflammatory cell infiltrate
tissue disruption
connective tissue deposition
chronic inflammation mainly mediated by what cells
macrophages and lymphocytes
cellular infiltrate consists of:
macrophages, lymphocytes, plasma cells, other leukocytes
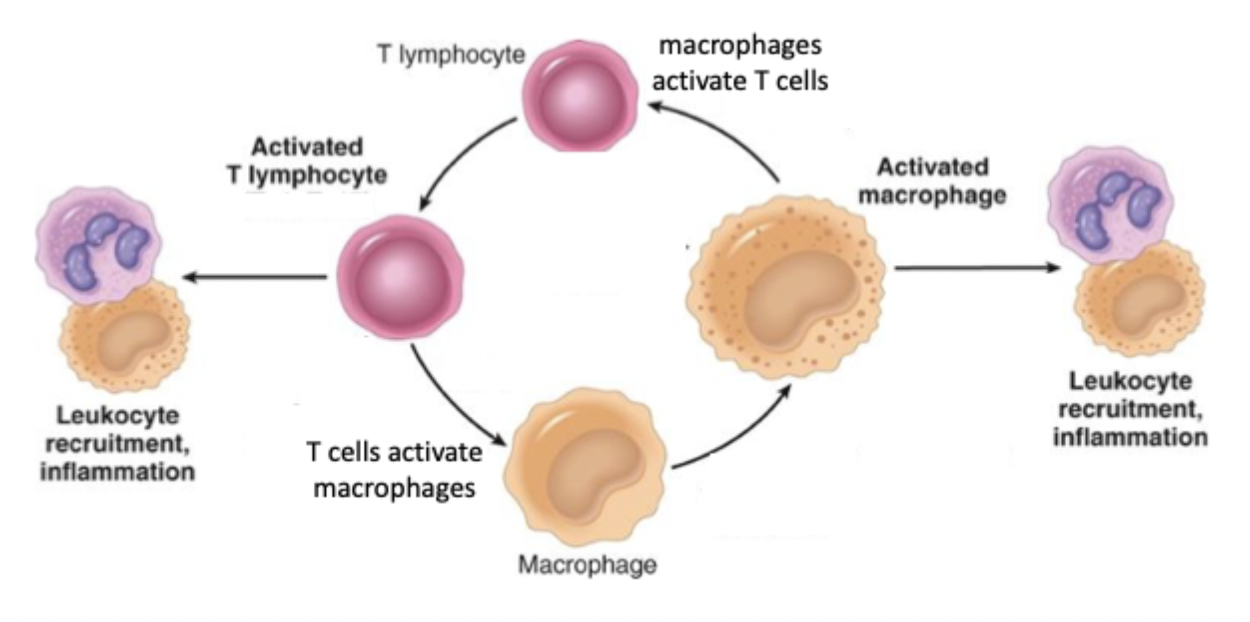
both cell types produce cytokines that drive inflamation and activate each other
macrophage/T cell bidirectional activation
other WBC involvement in chronic inflammation
B cell activation results in plasma cells that secrete antibody into the blood/tissue
antibody can bind to cells/cell receptors and trigger inflammation
mast clls accumulate in chronically inflamed tissue and secrete vasoactive an dpro-inflammatory mediators
eosinophils contribute to initiation and modulation of inflammation
granulomatous inflammation
distinct pattern of chronic inflammation
granuloma attempt to contain infecting agent
if caused by pathogen usually see a necrotic centre
centre surrounded by macrophages and epitheloid cells which are surrounded by lymphocytes
epitheloid cells can fuse to form large giant cells
epitheloid and giant cells are types of macrophages
conditions associated with granulomas
infectious disease: tuberculosis, leprosy, brucellosis, syphilis, mycotic infections
autoimmunity/autoinflammation: sarcoidosis, chrons disease