fatty acids and terpenoids
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
20 Terms
what are lipids and what are the two main classes of lipids (based on how they are derived)
biological molecules soluble in organic non-aqueous solvents
fatty acid derived lipids (C2 units)
terpenoid lipids (C5 units)
what is a fatty acid?
long chain carboxylic acids, often multiples of C2
describe triacylglycerols
three fatty acids ester bonded with gycerol alcohol groups
main use as storage molecule
lack of charged head group allows dense packing
describe glycerophospholipids
glycerol-3-phosphate with 2 ester bonded FAs
how are fatty acids transported?
via serum albumin
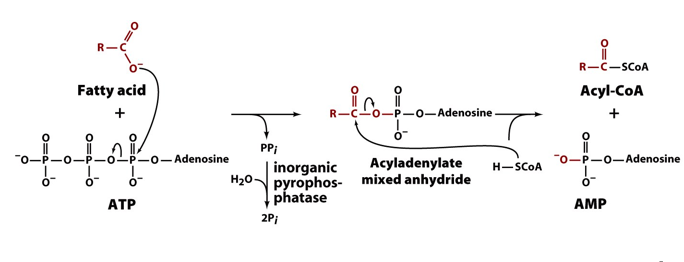
how are fatty acids activated?
FA attacks ATP to form an anhydride, where its acyl group is attached to AMP, and pyrophosphate leaves
CoA comes in and releases AMP by bonding with the terminal carbon of the fatty acid
forming acylCoA, which is active since it can now be oxidised
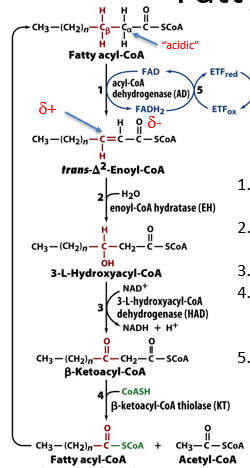
describe the beta degradation of fatty acids
FAD oxidises fatty acyl CoA into enoyl-CoA
this has resonance structures, where beta carbon is electrophilic, and attacked by water to make alcohol
NAD+ acts as hydride acceptor, making beta carbon into carbonyl
CoASH attacks beta carbon, causing release of acetylCoA and a fatty acylCoA that is shorter by 2 carbon atoms
special reactions are used to deal with unsaturated positions or uneven chain lengths or branching
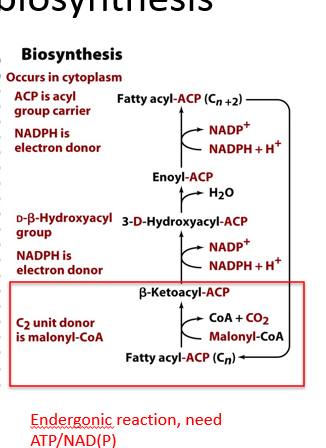
describe fatty acid biosynthesis
occurs in cytoplasm
C2 unit (malonyl) is added onto fatty acyl ACP to make ketoacylACP
keto group is reduced into alcohol (hydroxyacylACP)
alcohol group is dehydrated to make a double bond
double bond is reduced to make fatty acyl ACP (n+2)
ACP is acyl carrier protein. all synthesis steps occur on this protein, while all degradation steps occur on CoA.
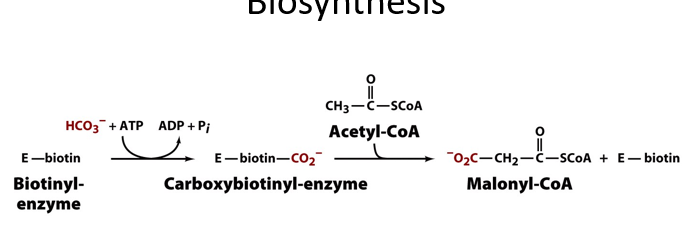
to make fatty acids, the first step required the C2 unit donor malonyl CoA. describe how this is made.
carboxylation of biotin-enzyme coupled withATP hydrolysis.
then substitute biotin-enzyme with acetyl CoA. this means youve ultimately just carboxylated acetyl CoA
driven by ATP
note that malonyl CoA has 3 carbon atoms
describe Biotin-dependent carboxylase enzymes (such as acetyl CoA carboxylase)
two active sites, BC= biotin Carboxylase, CT= carboxyl transferase
BCCP= biotin carboxyl carrier protein
bound to BCCP is a lysine attached to a biotin head molecule
the lysine acts as a flexible arm, can swing the biotin between active sites
tasnlaocation of biotin between active site has a distance of 55-85A
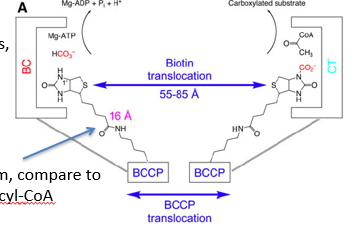
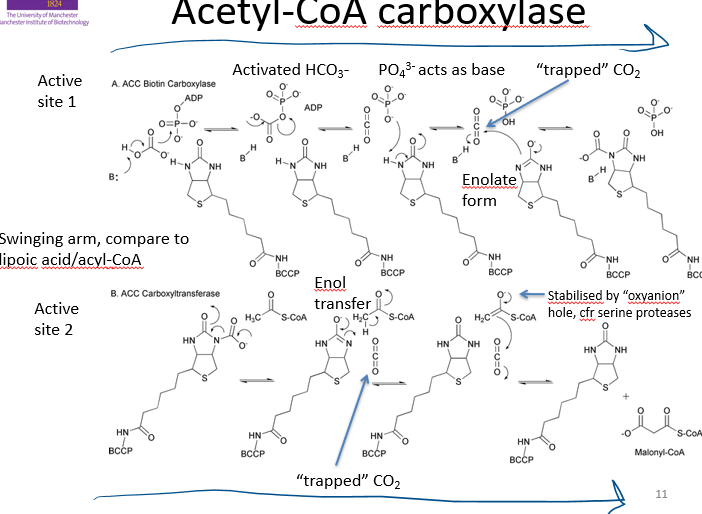
describe how the enzyme acetyl CoA carboxylase uses bccp
active site 1:
brings together carbonate (HCO3- ) and ATP
carbonate fuses to phosphate of ATP, which naturally falls apart into CO2 (which is trapped in enzyme due to crowding) and phosphate group
phosphate acts as a base, activates biotin to act as a nucleophile which accepts CO2
swing into active site 2:
CO2 released
nucleophilic biotin deprotonates 2C unit
2C unit now bonds with CO2 to make malonyl CoA
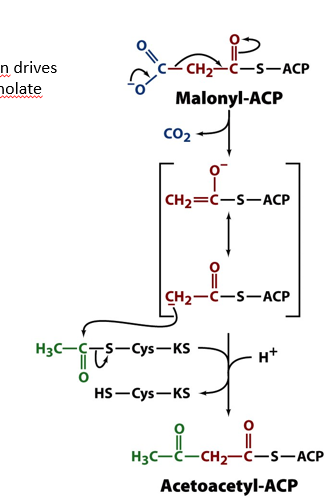
once malonyl is transferred from CoA to ACP using MAT enzyme, what happens?
malonyl is decarboxylated to provide nucleophilic carbon which attacks a carbonyl group from an enzyme (KS which has a cys residue bound to acetate via S)
what is the function of desaturase enzymes?
to make unsaturated fatty acids
compare the C2 (ketoacyl) building blocks we’ve just discussed to C5 building block
C5 building blocks are isoprene, mostly branched or cyclic products while ketoacyl blocks make mostly linear products
C-C elongation of 2C units uses carbanion chemistry, while elongation of 5C units uses carbocation based chemistry
describe hyperconjugation
the overlap of a p orbital on a carbocation with a neighbouring molecular orbital, like a C-H or C-C bond
stabilises the carbocation
describe the ranges of terpenoids
very prominent in plant metabolism, over 85k compounds, used in fragrances, hormones and toxins
how is diversity introduced to terpenoids?
by using different numbers of isoprene units as well as stopping chain elongation to allow folding
why are C5 units branched?
allow tertiary carbocation formation
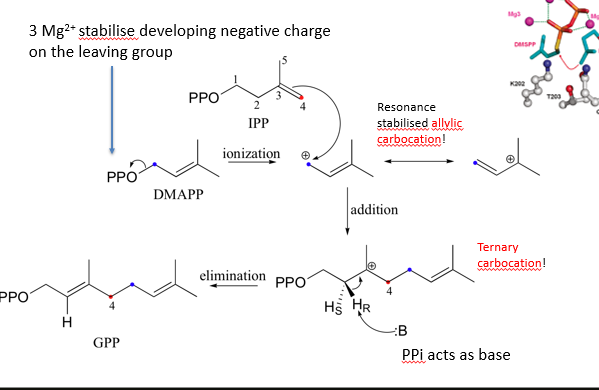
IPP isomerase enzyme. gives proton to C=C to form carbocation
should learn the ways in which tertiary carbocation is formed from the diagram
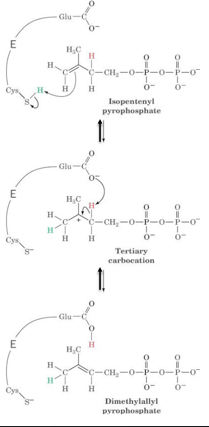
describe how a prenyltransferase attaches DMAPP to IPP
pyrophosphate of DMAPP leaves via sn1, making allylic carbocation (+ charge near C=C) which has resonance stabilisation
IPP is placed near carbocation, which is attacked by its electron rich C=C bond to make ternary carbocation
proton eleminated by PPi which acts as base
produces GPP, which acts similar to DMAPP, meaning the cycle can occur again with GPP used instead of DMAPP, thus chain elongates
magnesium required to allow PPi to leave at the start
what class of enzymes are responsible for the variety of terpenes?
terpene cyclases, which catalyse initial carbocation formation and substrate folding
small changes in their active site alter outcome of terpene