Biology - Chapter 3 An Introduction to Metabolism
1/84
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
85 Terms
metabolism
the sum of all chemical reactions in a cell or organism
the ability of a living organism to do work (move, grow, digest, transport sucrose across a cell membrane) requires
energy
2 main types of energy in biology
kinetic
potential
kinetic energy
occurs as a result of motion
potential energy
stored within an object
in biochemistry, this is often energy stored in chemical bonds
the total amount of energy in any closed system is
constant
energy cannot be _______ or _______. it can only be converted from _________ into _______
created, destroyed, one form, another
if a physical system gains an amount of energy, another physical system must
experience a loss of energy of the same amount
eg. photosynthesis converts light energy to chemical potential energy (carbs). this chemical energy is then consumed by animals and converted into high energy molecules (ATP), which is converted to other types of energy (thermal and kinetic)
however, the above example depends on chemical reactions where bonds are broken, and bonds are formed
when chemical bonds break
energy is absorbed
when chemical bonds form
energy is released
bond energy
the minimum amount of energy that is required to break a particular type of bond
every time energy is converted from one form to another, some of that energy is
lost (becomes unusable)
therefore, energy transfer is not 100% efficient
entropy
a measurement of disorder in a system
astronomers tell us that the Universe is continually increasing in entropy
what entropy means in terms of biology
when large particles are broken down into smaller particles (digestion)
when particles spread out (diffusion)
what entropy means in terms of chemistry
when solids react to form liquids or gases
when liquids react to form gases
when the total number of product molecules is greater than the total number of reactant molecules
spontaneous reactions
happens without requiring the input of energy from the cell
can only happen if the total amount of entropy increases
non-spontaneous reactions
whenever cells use energy (endocytosis), entropy decreases
cells use this energy to maintain order/organization
as spontaneous reactions proceed, __________ is released
free energy
free energy
available to perform work
spontaneous example
a brick wall ages and crumbles and falls apart into a random pile
in biology, this could be carbs being broken down into monosaccharides. this makes energy available to the cell (catabolic reaction)
entropy ______ because disorder _______
increases, increases
non-spontaneous example
a random pile of bricks. these bricks will not just build themselves into a wall. that will require energy.
in biology, this could be monosaccharides being built into carbohydrates (anabolic reaction)
disorder to order will _______ entropy
decrease
energy in sunlight for plants, food for animals maintains cell in a highly ordered state — the exact opposite of entropy. does this mean living organisms do not obey the second law of thermodynamics?
no, because the release of by-products such as thermal energy and CO2 increases the entropy of our surroundings, while the organisms themselves maintain order
in other words, the entropy of the organism decreases while the overall entropy of the Universe increases
ATP
adenosine triphosphate
ATP is the _____ for _________
energy carrier, almost all energy-driven actions in every cell on Earth
eg. mechanical work (beating of cilia or the contraction of muscle fibres)
transport work (pumping substances across membrane against a concentration gradient)
chemical work (protein synthesis)
what is ATP made of
nitrogenous base called adenine is linked to:
a 5-carbon sugar called ribose is liked to:
a chain of 3 phosphate groups
what does the 3 phosphate groups being crowded together do?
their close proximity creates a mutual repulsion of their electrons
similar to the potential energy in a compressed spring
the last phosphate wants to split off from the others and the result is a release of the potential energy stored in the bond
the terminal phosphate is easily broken off by a hydrolysis reaction to form ADP (adenosine diphosphate) and inorganic phosphate
why is energy released when a bond is broken?
breaking off the terminal phosphate is energetically favourable because of the repulsion between the phosphates
the entropy of the system is increased
1 molecule split into 2 molecules
the H and the OH from the water molecules form 2 new bonds with the ADP and the inorganic phosphate
overall the reaction releases energy, about 7.3 Kcal/mol of energy is released
ATP is _______ to form ______ and ______
hydrolysed, ADP, inorganic phosphate
energy is released during this reaction
to convert ADP back into ATP, _________ reaction is used and the ________ is _________
the opposite, a phosphate is added back onto the ADP
requires energy
energy comes from the food we eat, carbs, fats, proteins
why is ATP called the universal energy ‘currency’
it directly supplies the energy that powers nearly every cellular function
the types of work carried out by ATP includes ______, ______, and ______
mechanical, transport, and chemical work
mechanical work
beating of cilia or movement of flagella
contraction of muscle fibres
movement of chromosomes during mitosis/meiosis
transport work
process of pumping substances across membranes against their concentration gradient
chemical work
process of supplying chemical potential energy for non-spontaneous, endergonic reactions, including protein synthesis and DNA replication
phosphorylation
the transfer of a phosphate group, usually from ATP, to another molecule
how is ATP regenerated
the Pi is synthesized back onto the ADP with the addition of free energy
why is ATP used as the universal energy currency rather than cells using the energy, for example, from food?
cells use ATP as an immediate source of energy because it has specific properties that are important for the biochemical reactions that allow proper cell functioning
complex food molecules also require numerous reactions to release their energy, but ATP can be created and accessed immediately
enzyme
a biological catalyst (usually a protein) that speeds up a chemical reaction
approximately how many enzymes are present in a typical cell
about 4000 different enzymes in a typical living cell
what must happen for a chemical reaction to move forward
it must overcome an energy barrier
what do enzymes do to help this process
enzymes bind a specific reactant (or reactants), called a substrate; in doing so, they lower the energy barrier so that the reaction proceeds at a faster rate than it would without the enzyme
substrate
a substance that is recognized by and binds to an enzyme
active site
a pocket or groove in an enzyme that binds its substrate
induced fit model
a model of enzyme activity that descries how an enzyme changes shape to better accommodate a substrate
why are enzymes able to change shape in order to make the active site even more precise
because they are not rigid objects but are flexible
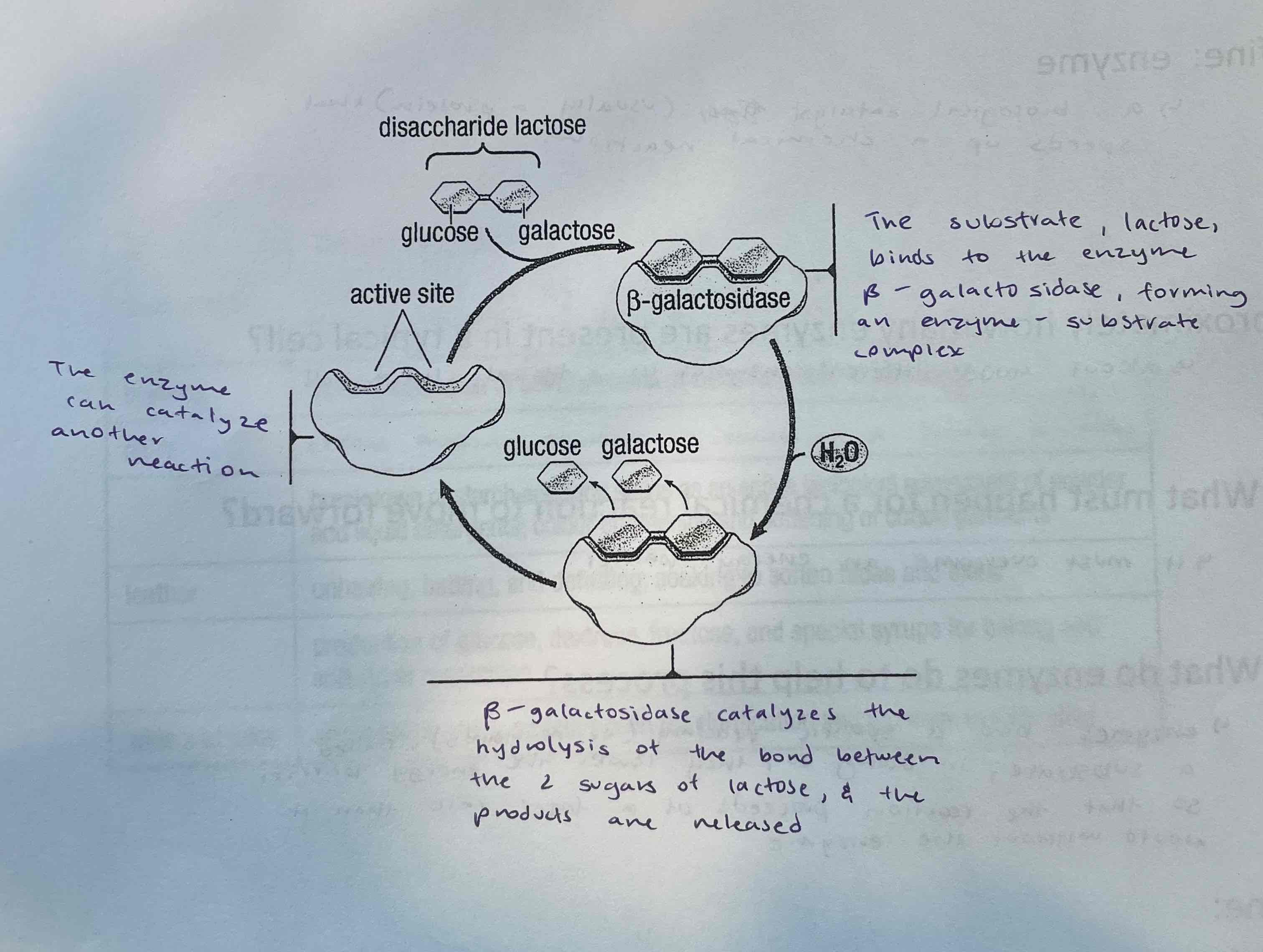
catalytic cycle
cofactors
non-protein group that binds precisely to an enzyme
often nonmetals (iron, copper, zinc, manganese)
essential for catalytic activity
eg. an enzyme that is essential for providing one of the key components of the chemical pathway within mitochondria for the production of energy requires a magnesium cofactor to function properly
coenzyme
organic cofactors
derive from water-soluble vitamins
many coenzymes shuttle molecules from one enzyme to another
ex. NAD+ , a derivative of vitamin B2 acts as a electron carrier during a number of biochemical pathways
enzyme inhibitors
molecules that bind to an enzyme and decrease its activity, lowering the rate at which an enzyme catalyzes a reaction
competitive inhibition
a situation in which a competitor substance binds to a normal substrate binding site to block enzyme activity
the inhibitor actually competes with the normal substrate for access to the active site of the enzyme
noncompetitive inhibition
a situation in which molecules bind to an enzyme at a site that is not the active site, thus blocking enzyme activity
non-competitive inhibitors bind to an enzyme at a location other than the active site. this changes the shape of the enzyme, reducing the ability of the substrate to bind efficiently
molecules that naturally regulate enzyme activity in a cell often behave like
a noncompetitive reversible inhibitor
these molecules bind to a special site on the enzyme called an
allosteric site
this causes a change in
the shape of the enzyme, thus affecting the active site
this type of regulation is called
allosteric regulation
feedback inhibition
the regulation of a pathway by one of the products of this pathway
what happens in feedback inhibition
the regulation of the pathway by one of the products in the pathway
feedback inhibition happens in the pathway that produces ________ from _________.
isoleucine, threonine
product of the feedback inhibition pathway
isoleucine
what happens if isoleucine accumulates in excess
it slows or stops the pathway by acting as an allosteric inhibitor of the enzyme that catalyzes the first step in the pathway
enzyme activity is significantly altered by changes in
pH and temperature
what happens when there are deviations from an enzyme’s optimal conditions
leads to decreased activity, often represented by a peaked curve in activity graphs
each enzyme has an optimal pH for peak efficiency, usually around ____ for cellular processes
pH 7.5
what happens when an enzyme’s pH deviates from it’s optimal amount?
the enzyme’s activation site is severely impacted, and potentially reduces reaction rates to 0
enzymes secreted from cells may have ________ pH optima
varying
pepsin optimal pH level
1.5 (stomach acidity)
trypsin optimal pH level
8 (alkaline intestinal environment)
temperature influences enzyme activity through __ main processes
2
general reaction rate
higher temperatures increase reaction rates due to increased molecular motion and collision frequency
protein structure
higher temperatures enhance the kinetic motion of amino acid chains in structures, but excessive heat leads to denaturation, breaking the enzyme’s 3D structure
enzyme activity generally ______ for every ___ increase in temperature from ____ to _____
doubles, 10°C, from 0°C to 40°C
what happens when the temperature in an enzyme’s environment goes beyond 40°C
it begins to denature, leading to a decrease in activity
for most enzymes, the peak in activity lies between
40°C to 50°C
for most enzymes, activity sharply declines at ____ and reaches 0 around _____
55°C, 60°C
effects of enzymes on animal feed
degradation of the components to feed to improve nutrient digestion and uses of the feed
effects of enzymes on brewing
faster maturation of beer; removal of carbohydrates in lighter beer
effects of enzymes on dairy
cheese making; removal or conversion or lactose in milk
effects of enzymes on detergent
breakdown of starch and fatty stains as an active biological component of powder and liquid detergents; colour brightening and softening of cotton garments
effects of enzymes on leather
unhairing, batting, and defatting; soaking to soften hides and skins
effects of enzymes on starch
production of glucose, dextrose, fructose, and special syrups for baking and soft-drink production
wine and juice
degradation of the protein pectin for clarification and increase in juice yield