hydrogen-oxygen fuel cells
1/5
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
6 Terms
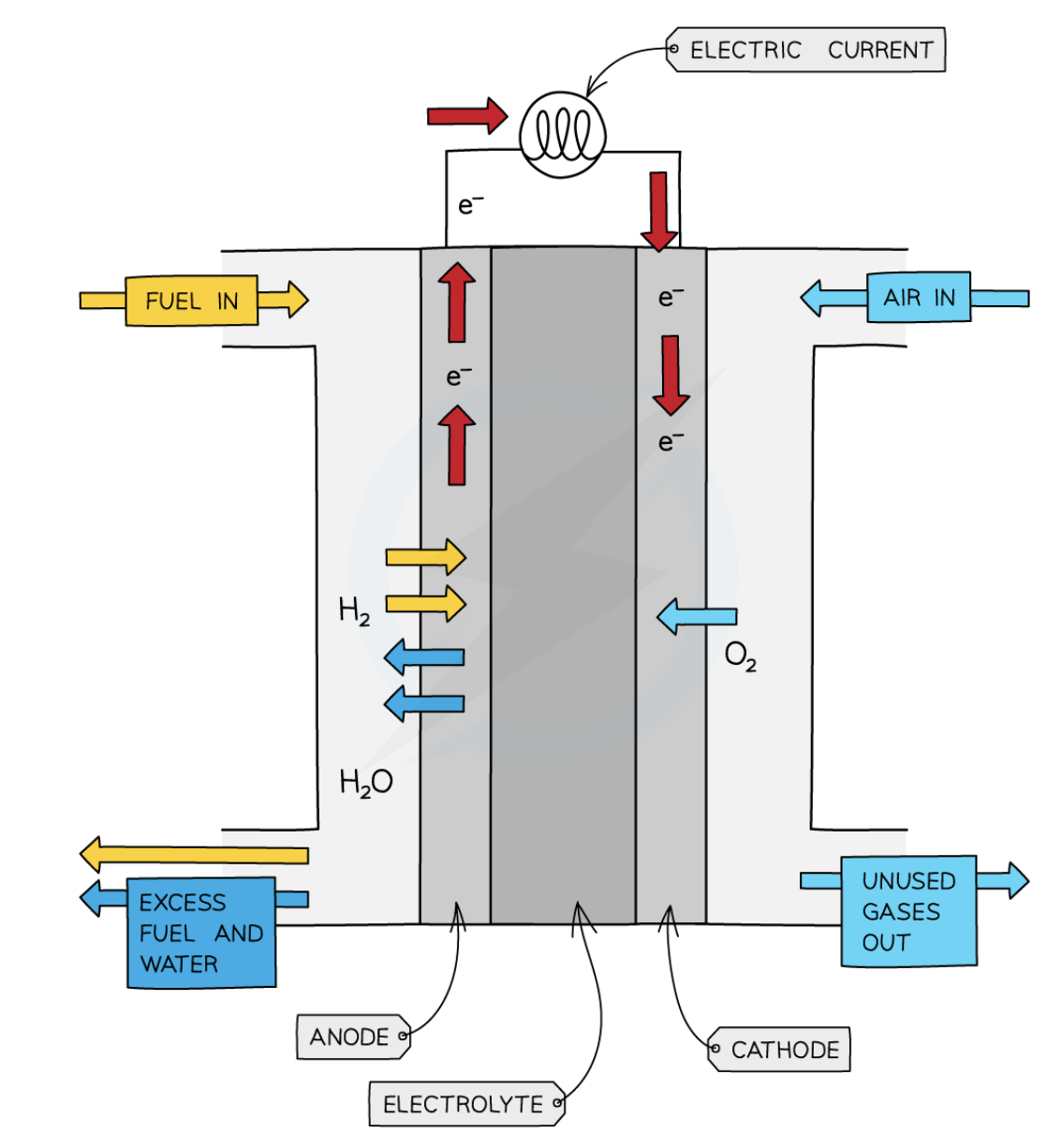
energy transfer
chemical → electrical
equation for hydrogen fuel cell reaction
2H2 + O2 → 2H2O
gases used to generate electricity in a fuel cell
hydrogen, oxygen
fuel cell
advantages of hydrogen fuel cells
hydrogen can be produced by water so its renewable
does not produce any pollution (only produces water. theres no CO2)
more energy per kg than petrol or diesel
quieter so less noise pollution compared to a petrol engine
disadvantages of a hydrogen fuel cell
electrolysis of water requires large amounts of energy
materials used to make fuel cells are expensive
hydrogen is more difficult and expensive to store (highly flammable and easily explodes under pressure)
fuel cells are affected by low temperatures (less efficient)
small number of hydrogen filling stations across the country