College Chemistry Chapter #2 Exam Study
1/11
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
12 Terms
Dalton's Postulates:
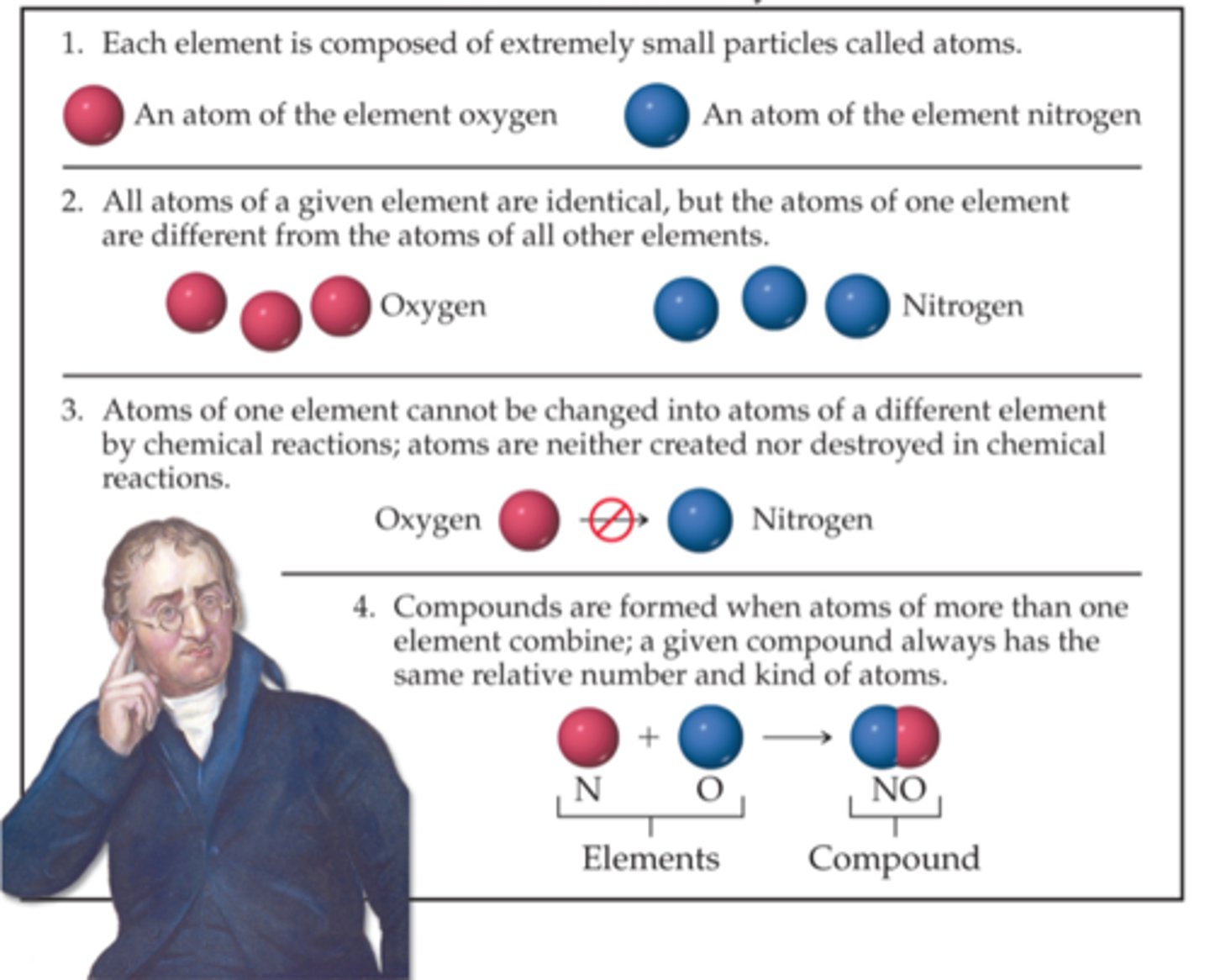
Law of Definite Proportions:
States that regardless of the amount, a pure compound always contains the same elements in the same proportions by mass.
Law of Conservation of Matter:
The total mass of materials present after a
chemical reaction is the same as the total
mass present before the reaction.
Charge/mass ratio of the electron:
1.76 x 10 e(8) C/g
Mass Number is equal to:
Protons + Electrons
Isotopes are an element with differing:
(A.) Protons
(B.) Electrons
(C.) Neutrons
(C.) Neutrons
___________ formulas give the exact number of
atoms of each element in a compound.
Molecular
___________ formulas give the lowest whole-number
ratio of atoms of each element in a compound.
Empirical
____________ formulas show the order
in which atoms are bonded.
Structural
Polyatomic ions are
composed of more than one
______.
Atom
In Ionic Compounds, ________ are usually a metal.
Cations
In Ionic Compounds, ________ are usually a non-metal.
Anions