Chem 211 and 213 Flashcards (Summer 2025)
1/91
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
92 Terms
Chemistry Definition
Study of matter, its properties, its changes, and energy associated with those changes. (Week 1 Material)
Sig Fig rules
Read the number left to right. Begin with the first nonzero digit.
Nonzero digits are ALWAYS significant.
Leading zeroes are never significant
Zeroes in between other nonzero numbers are significant.
Trailing zeroes are significant only if the number contains a decimal point
For example, 14,000. has 5 sig figs but 14,000 only has 2.
Exact numbers have infinite sig figs. Stuff that you can count. Like 35 mL has infinite sig figs. Its an exact integer.
When looking at a number in scientific notation, you only look at the coefficient when counting sig figs.
For multiplication/division, you will round your answer to the number of sig figs as the least factor.
(Week 1 Material)
Law
A relationship between variables. Laws don't give explanations. (Week 1 Material)
Theory
A unifying principle that explains a body of facts and or those laws that are based on them. (Week 1 Material)
Matter
Anything that takes up space and has mass (Week 1 Material)
Pure Substances
Matter that cannot be separated into other kinds of matter by physical processes. Can be elements or compounds. (Week 1 Material)
Mixtures
Matter which can be separated into other kinds of matter by physical processes. (Week 1 Material)
Homogenous Mixture (Solution)
A mixture that has uniform properties throughout. For example, clean air. (Week 1 Material)
Heterogeneous mixture
Mixture that consists of physically different parts (Week 1 Material)
Elements
Substance which cannot be decomposed into simpler substances by chemical means. (Week 1 Material)
Classifications of matter
(Week 1 Material)
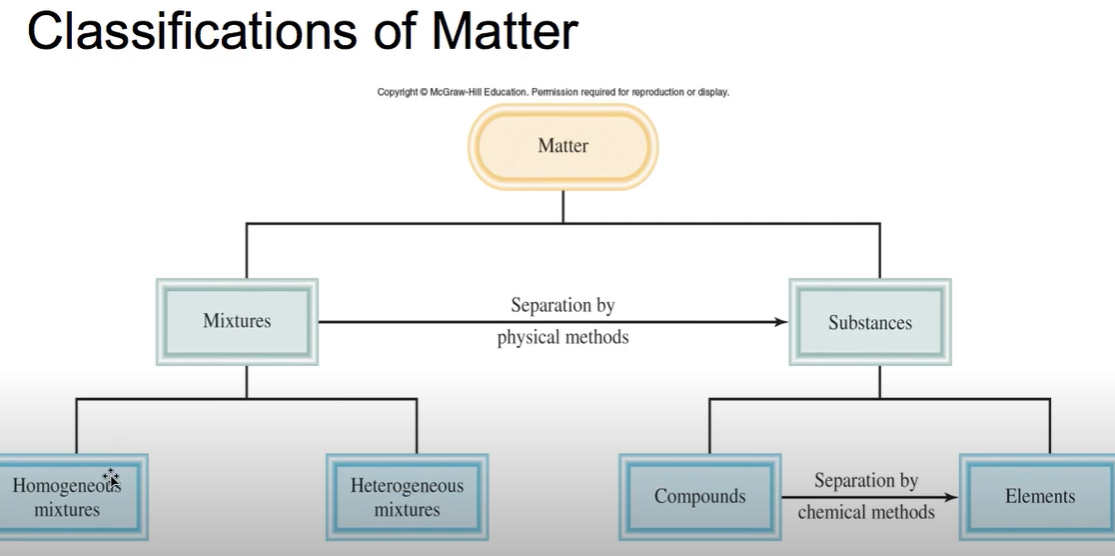
Physical Change
Does not alter the composition or identity of the substance, an example is ice melting (Week 1 Material)
Chemical Change
Does alter the composition or identity of the substance. (Week 1 Material)
Extensive Property
Depends on how much matter there is. Like mass, length, volume (Week 1 Material)
Intensive Property
Does NOT depend on how much matter there is. Like density, temperature, color. (Week 1 Material)
Seven basic SI units
Any variable can be found with these seven. For example, distance over time is velocity. (Week 1 Material)
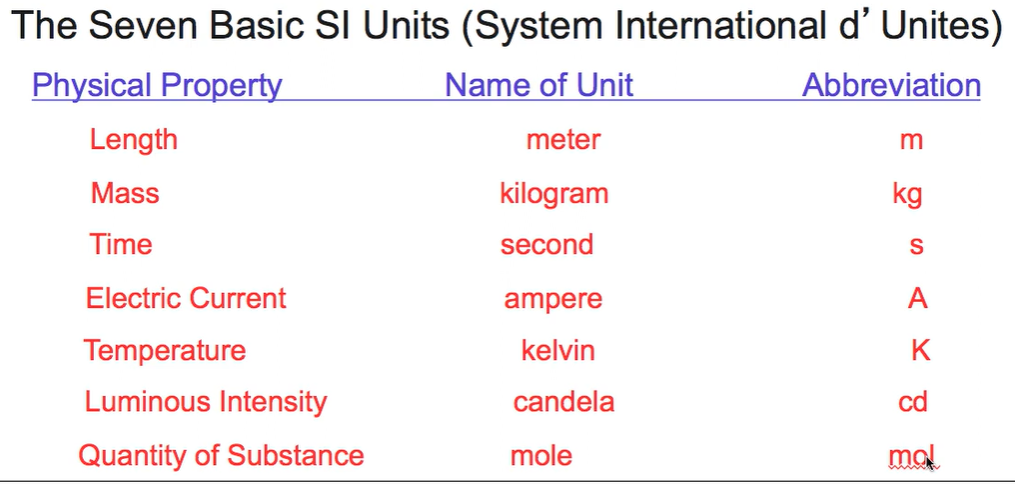
When you read measurement instruments, too how many digits can you report your answer?
1 more than the finest digit shown. (Week 1 Material)
Systematic Errors
Errors that make the experimental results different from the true value with reproductive discrepancies. Affects the accuracy of the result. (Week 1 Material)
Random Errors
Fluctuations in observations that differ from experiment to experiment and require repeated measurements to yield precise results. (Week 1 Material)
Accuracy
A measure of how close the result is to the true value. (Week 1 Material)
Precision
Measure of the reproducibility of the result. How close each measurement is too each other. (Week 1 Material)
Density formula
Rho is density, m is mass and V is volume. (Week 1 Material)
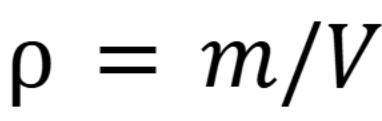
Celsius to Fahrenheit formula
F = 1.8C + 32 (Week 1 Material)
Law of constant composition
Compounds always contain the same mass percent per element. (Week 1 Material)
Law of multiple proportions
The different mass ratios of elements A and B are related by the ratio of two small whole numbers. For example, Phosphorus chloride I and phosphorus chloride II, the ratio is 5:3. (Week 1 Material)
John Daltons Atomic Theory
1. Elements are composed of small particles called atoms
2. All atoms of a given element are identical, same size, mass and chemical properties. The atoms of one element are different from atoms of other elements.
3. Compounds are composed of atoms of more than one element
4. A chemical reaction involves only the separation, combination, or rearrangement of atoms. Not destruction.
(Week 1 Material)
Who discovered the electron and could determine the mass charge ratio
J.J. Thomson (Week 1 Material)
Would conducted the oil drop experiment to determine the charge of an electron
Robert Milikian (Week 1 Material)
Charge of electron
(Week 1 Material)
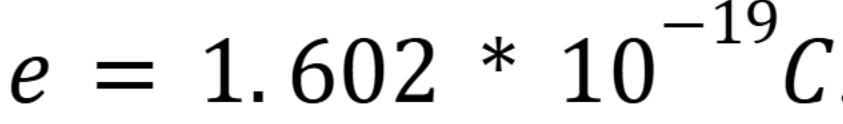
What is in the nucleus of atoms
Protons and neutrons (Week 1 Material)
What are rows and columns known as in the periodic table
Rows = Periods. Columns = Groups (Week 1 Material)
Periodic Table
(Week 1 Material)
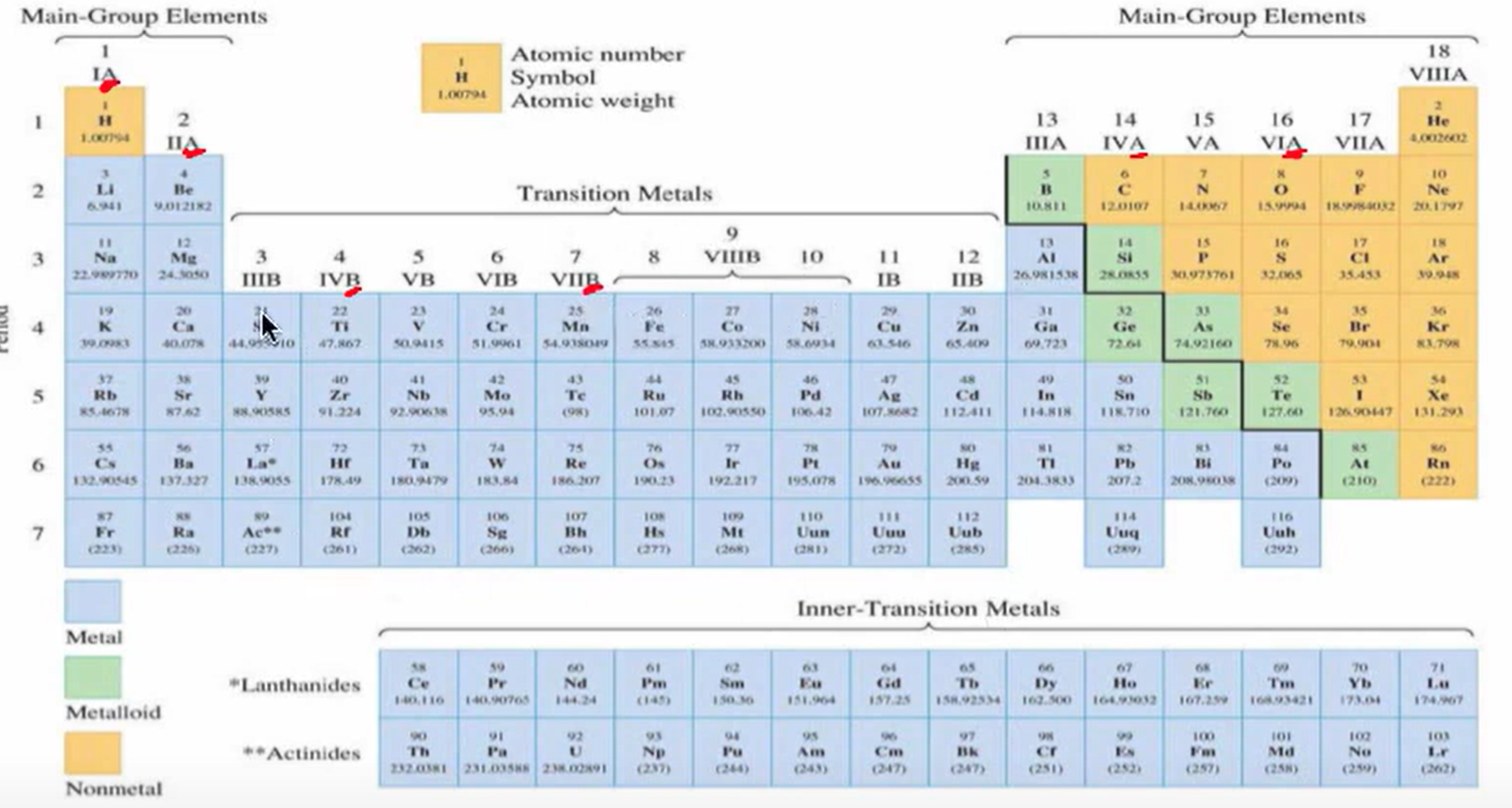
What are the naturally occurring diatomic elements.
Hydrogen, nitrogen, oxygen, fluorine, chlorine, bromine, and iodine (Week 1 Material)
What are the two common ways atoms chemically combine
Ionic bonds or covalent bond (Week 1 Material)
Ionic Bonds
Atoms transfer electrons from atoms of one element to another. Like NaCl is an ionic compound. Its a bond between ions. Very common between metals and non-metals. There's an electric force. Since they transfer electrons, there will be a positive atom and a negative one. Opposite charges attract. (Week 1 Material)
Covalent Bonds
Electrons are shared. Occurs often in non-metals like water. (Week 1 Material)
Cation
Ion with positive charge (Week 1 Material)
Anion
Ion with negative charge (Week 1 Material)
Metals tend to do what with electrons
Lose them. Hence a positive charge (Week 1 Material)
Empirical formula
Simplest compound possible (Week 1 Material)
What is NO3 called. What's its charge
Nitrate. -1 charge (Week 1 Material)
What is NO2 called. What's its charge
Nitrite. -1 charge (Week 1 Material)
What is SO4 called. What's its charge
Sulfate. -2 charge (Week 1 Material)
What is SO3 called. What's its charge
Sulfite. 2- charge (Week 1 Material)
What is PO4 called. What's its charge
Phosphate. -3 charge (Week 1 Material)
What is PO3 called. What's its charge
Phosphite. -3 charge (Week 1 Material)
What is NH4 called. What's its charge
Ammonium. +1 charge (Week 1 Material)
What is C2H3O2 called. What's its charge
Acetate. -1 charge (Week 1 Material)
What is OH called. What's its charge
Hydroxide. -1 charge (Week 1 Material)
What is MnO4 called. What's its charge
Permanganate. -1 charge (Week 1 Material)
What is ClO3 called. What's its charge
Chlorate. -1 charge (Week 1 Material)
What is ClO2 called. What's its charge
Chlorite. -1 charge (Week 1 Material)
What is CrO4 called. What's its charge
Chromate. -2 charge (Week 1 Material)
What is Cr2O7 called. What's its charge
Dichromate. -2 charge (Week 1 Material)
What is HSO4 called. What's its charge
Hydrogen sulfate. -1 charge (Week 1 Material)
What is CO3 called. What's its charge
Carbonate. -2 charge (Week 1 Material)
What is HCO3 called. What's its charge
Hydrogen carbonate. -1 charge. (Week 1 Material)
What is ClO4 called. What's its charge.
Perchlorate. -1 charge (Week 1 Material)
For binary molecular compounds (Usually two non metals) what suffix is used at the end of the second element? Do you ever use the prefix mono for the second element?
Use the suffix -ide for the second element. Never use Mono. (Week 2 Material)
Prefixes for the number of atoms for the first element: 1
Mono (Week 2 Material)
Prefixes for the number of atoms for the first element: 2
Di (Week 2 Material)
Prefixes for the number of atoms for the first element: 3
Tri (Week 2 Material)
Prefixes for the number of atoms for the first element: 4
Tetra (Week 2 Material)
Prefixes for the number of atoms for the first element: 5
Penta (Week 2 Material)
Prefixes for the number of atoms for the first element: 6
Hexa (Week 2 Material)
Prefixes for the number of atoms for the first element: 7
Hepta (Week 2 Material)
Prefixes for the number of atoms for the first element: 8
Octa (Week 2 Material)
Prefixes for the number of atoms for the first element: 9
Nona (Week 2 Material)
Prefixes for the number of atoms for the first element: 10
Deca (Week 2 Material)
Acids are in what form
H___(aq) (Week 2 Material)
What are the two types of acids
Binary acids, and Oxyacids (Week 2 Material)
Binary acids
Acids that have just a nonmetal with them. An example would be HCl (aq) (Week 2 Material)
Oxyacids
Acids that have polyatomic anions with them, (Nonmetal and oxygen). An example would be HNO3 (Week 2 Material)
How do you name binary acids
Use the prefix hydro, the element name(ic) acid. For example, HCl is Hydrochloric acid. (Week 2 Material)
How do you name Oxyacid
You DONT use the prefix hydro. The suffix -ic and -ous always belongs to the acid. When going from acid to ion, its -ic to -ate, and -ous to -ite. (Week 2 Material)
What is 1 mol
6.022*10^23 (Week 2 Material)
Empirical formula
The simplest formula for a compound. For example, glucose's formula is C6H12O6. So its empirical formula would be CH2O (Week 2 Material)
Molecular formula
The formula for the compound as it exists. For example, glucose is C6H12O6 (Week 2 Material)
Theoretical yield
Maximum amount of product that can be obtained from the reactants (Week 2 Material)
Actual yield
Actual amount of product obtained in real life from the reactants. (Week 2 Material)
Percent yield
(Actual Yield/Theoretical Yield)*100 (Week 2 Material)
Limiting Reagent
Reactant that is entirely consumed during a reaction, thereby limiting the amount of product that can be produced. (Week 2 Material)
How do you determine the limiting reagent
Whichever reactant has a mol/coefficient ratio that's smallest, that's the limiting reagent (Week 2 Material)
Solution
Solute and solvent (Week 2 Material)
Molarity (M) equation
Moles of solute/liters of solution (Week 2 Material)
Molality (m) equation
Moles of solute/kilograms of solvent. (Week 2 Material)
Mass percent equation
Mass of solute/mass of solution (Week 2 Material)
Dilution equation
M is Molarity, V is volume. (Week 2 Material)
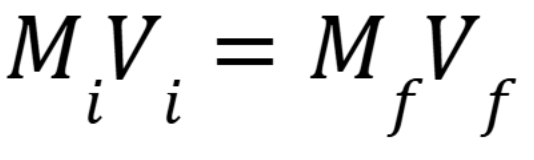
Solubility
Maximum amount of solute that will dissolve in a certain solvent at a specific temperature (Week 3 Material)
Solubility Rules
Rules 1 and 2 are MOST important (In water at 25 Celsius) (Week 3 Material)
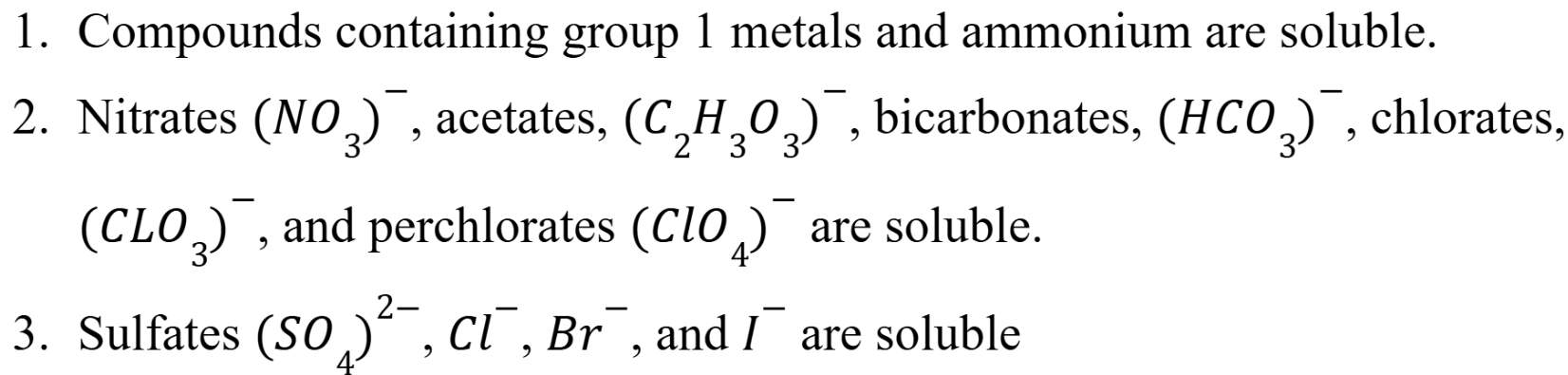
Insolubility Rules
(In water at 25 Celsius) (Week 3 Material)
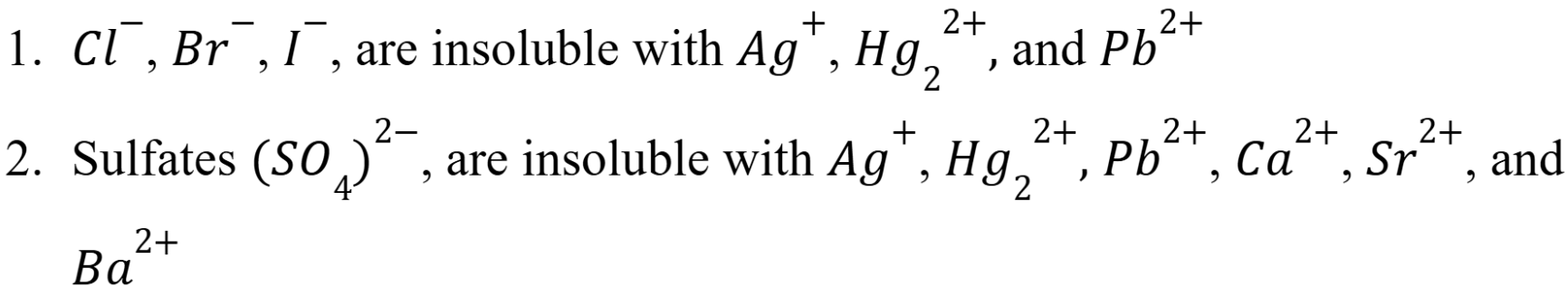
Kinetic Molecular Theory