Ochem 1 - pKa Values of Acids/Bases
1/33
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
34 Terms
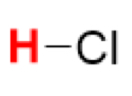
pKa of HCl
(Hydrochloric Acid)
-7
What is the Conjugate base of:
H—Cl
(Hydrochloric Acid)
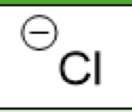
Cl-
(Chloride)
Acid or Base?
HCl
Strong Acid
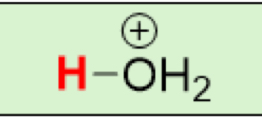
pKa of H₃O⁺
(Hydronium)
-1.7
Acid or Base?
H₃O⁺
Strong Acid
What is the Conjugate Base of:
H₃O⁺
(Hydronium)
H₂O (water)
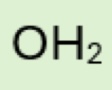
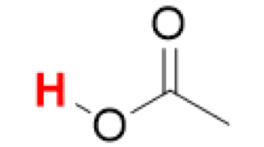
pKa of CH₃COOH
(Acetic Acid)
4.8
Acid or Base?
CH₃COOH
Acetic acid
Strong Acid
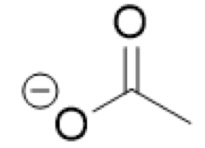
What is the Conjugate base of:
CH₃COOH
Acetic acid
CH₃COO- (acetate)
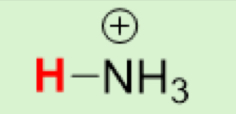
pKa of NH₄⁺
(Ammonium)
9.25
Acid or Base?
Ammonium (NH₄⁺)
Strong Acid
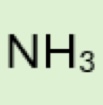
What is the Conjugate base of:
Ammonium (NH₄⁺)
NH₃ (ammonia)
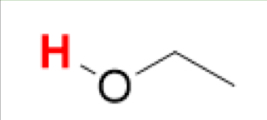
pKa of CH₃CH₂OH
(Ethanol)
16
Acid or Base?
CH₃CH₂OH
(ethanol)
Neutral (Able to donate protons)
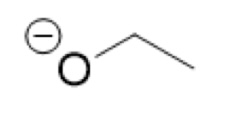
What is the Conjugate Base of:
CH₃CH₂OH
(Ethanol)
CH₃CH₂O- (ethoxide / alkoxide)
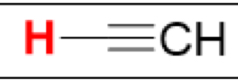
pKa of HC≡CH, sp
(Ethyne)
25
Acid or Base?
HC≡CH, sp
(Ethyne)
Weak Acid
What is the Conjugate Base of:
HC≡CH, sp
(Ethyne)
HC≡C- (acetylide anion)


pKa of H₂
Hydrogen
35
Acid or Base?
H₂
Hydrogen
Neutral
What is the Conjugate base of:
H₂
Hydrogen
H- (hydride)
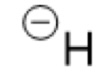
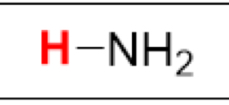
pKa of NH₃
(Ammonia)
38
Acid or Base?
NH₃
(Ammonia)
Weak Base
What is the Conjugate Base of:
NH₃
(Ammonia)
NH₂- (amide)
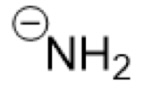
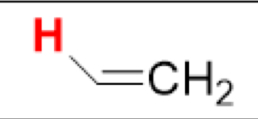
pKa of (CH₂=CH₂, sp²)
(Ethene)
44
Acid or Base?
(CH₂=CH₂, sp²)
(Ethene)
Neutral
(weak acid/weak base)
What is the Conjugate Base of:
(CH₂=CH₂, sp²)
(Ethene)
-CH=CH₂ (ethenide)
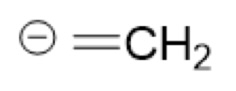
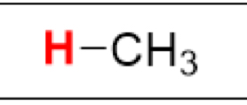
pKa of (CH₄, sp³)
(Methane)
50
Acid or Base?
(CH₄, sp³)
(Methane)
Neutral
(weak acid/weak base)
What is the Conjugate Base of:
(CH₄, sp³)
(Methane)
-CH₃ (carbanion)
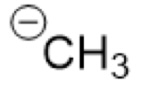
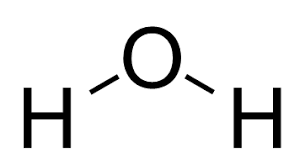
pKa of H2O
(Water)
15.7
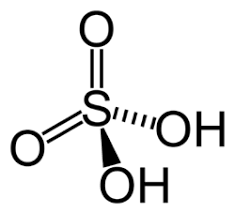
pKa of H2SO4
(Sulfuric Acid)
-3
Acid or Base?
H2SO4
(Sulfuric Acid)
Strong Acid
What is the Conjugate Base of:
H2SO4
(Sulfuric Acid)
HSO4- (Hydrogen Sulfate Ion)