Understanding Gas Laws: Boyle's, Charles', and Dalton's
1/152
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
153 Terms
What is pressure in the context of gas laws?
Pressure is the force per unit area caused by molecular collisions on the sides of the container.
What happens to the volume of a gas as external pressure increases, according to Boyle's Law?
As the external pressure on the gas increases, the volume of the gas decreases.
What variables are kept constant in the syringe experiment related to Boyle's Law?
Temperature and the number of moles of gas.
What type of relationship exists between pressure and volume in Boyle's Law?
An inverse relationship, meaning as one variable increases, the other decreases.
Explain what an inverse relationship means in the context of pressure and volume.
An inverse relationship means that as the pressure increases, the volume decreases, and vice versa.
What happens to gas particles in a syringe when the volume decreases as pressure increases?
The gas particles are compressed into a smaller volume while the number of particles remains constant.
Why does pressure inside the syringe increase as the volume decreases?
With constant temperature and moles, a decrease in volume results in an increase in pressure to maintain the relationship of P multiplied by V.
What happens to a balloon's volume when transported from a high-pressure area to a low-pressure area, such as from Ohio to the top of Mt. Everest?
As the balloon rises, the pressure decreases, causing the volume of the balloon to increase.
What is Boyle's Law expressed mathematically?
Boyle's Law is expressed as P1V1 = P2V2.
How can Boyle's Law be useful in real-life applications?
If you know the pressure and volume of a gas, you can predict the new volume if the pressure changes.
Calculate the new pressure of an ear drum volume change using Boyle's Law: If P1 is 760 mmHg and V1 is 0.54 cm³, what is P2 when V2 is 0.57 cm³?
P2 is 720 mmHg, calculated using P1V1 = P2V2.
What is the initial volume and pressure of a helium balloon before it rises?
The initial volume is 2.5 L at a pressure of 760.0 mmHg.
What is the pressure at 15,000 feet where a helium balloon rises?
The pressure at 15,000 feet is 415.0 mmHg.
Using Boyle's Law, how would you find the new volume of a helium balloon at 15,000 feet?
Use the formula P1V1 = P2V2 to solve for the new volume.
What is the significance of Boyle's Law in understanding gas behavior?
It helps predict how gases will behave under changing pressure and volume conditions.
What experimental setup is used to study Boyle's Law?
A syringe with a gas where pressure is varied while keeping temperature and moles constant.
In Boyle's Law experiments, what happens to the gas volume when the pressure is doubled?
The volume of the gas is halved.
What is the relationship between pressure and volume in a closed system according to Boyle's Law?
In a closed system, pressure and volume are inversely proportional.
What is the effect of temperature on the relationship described by Boyle's Law?
Boyle's Law assumes constant temperature; if temperature changes, the relationship may not hold.
How does Boyle's Law apply to everyday phenomena, such as ear pressure changes during flights?
It explains how changes in altitude affect the pressure and volume of gases in the body, like in the ear drum.
What is the role of molecular collisions in determining gas pressure?
Molecular collisions exert force on the walls of the container, contributing to the overall pressure.
What is the significance of keeping the number of moles constant in Boyle's Law experiments?
It ensures that the relationship between pressure and volume can be accurately observed without interference from changing gas amounts.
What is the relationship expressed by the equation P1V1 = P2V2?
It represents Boyle's Law, which states that the pressure and volume of a gas are inversely related when temperature is constant.
What happens to the volume of a gas if the pressure is doubled?
The volume will decrease by half.
What is kinetic energy in the context of gas particles?
Kinetic energy is the energy of motion of particles.
How is temperature related to kinetic energy?
Temperature is a relative measure of the average kinetic energy of particles.
What is the Kelvin scale based on?
The Kelvin scale is based on absolute zero, the temperature at which there is no molecular motion.
What is absolute zero in Kelvin?
Absolute zero is 0 K.
What occurs to the volume of a gas when its temperature decreases, according to the balloon experiment data?
As the temperature of the gas decreases, the volume of the balloon decreases.
What properties are kept constant in the balloon experiment measuring volume and temperature?
Pressure and the number of moles of gas are kept constant.
What type of relationship exists between volume and temperature in Charles's Law?
The relationship is direct; as temperature increases, volume increases.
What happens to gas particles when the temperature decreases?
The particles lose energy, move less, and the volume of the gas decreases.
How do you convert Celsius to Kelvin?
To convert Celsius to Kelvin, add 273 to the Celsius temperature.
Why are there no negative temperatures on the Kelvin scale?
There are no negative temperatures because absolute zero (0 K) represents the absence of energy.
What is Charles's Law?
Charles's Law states that the volume of a gas is directly proportional to its temperature in Kelvin when pressure is constant.
How can Charles's Law be useful?
It allows prediction of a gas's volume at different temperatures, provided the temperature is in Kelvin.
If a gas occupies 1400 m³ at 83°C, what will its temperature be at 1200 m³?
The temperature will be 32°C.
What is the formula used to calculate the new temperature in Charles's Law?
The formula is T2 = (V2 * T1) / V1.
What is the initial temperature in Kelvin for a gas at 83°C?
The initial temperature is 356 K.
What happens to the volume of a gas when the temperature is increased?
The volume increases if pressure is held constant.
What is the significance of the Kelvin scale in gas law calculations?
The Kelvin scale is essential because gas laws require absolute temperature for accurate calculations.
What is the volume of the gas at 4.0°C according to the balloon experiment data?
The volume is 1.41 L.
What is the volume of the gas at -3.0°C according to the balloon experiment data?
The volume is 1.37 L.
What happens to the volume of a gas when the temperature reaches absolute zero?
The volume would theoretically reach zero, as there would be no molecular motion.
What is the formula used to calculate the new volume of a gas when temperature changes according to Charles' Law?
(V1)(T2) = (V2)(T1) or V2 = (V1)(T2) / T1.
What are the initial conditions for the gas in the syringe before warming?
V1 = 25.0 mL, T1 = 22°C (295 K).
What is the final temperature of the gas when it warms up outside?
T2 = 32°C (305 K).
What is the new volume in the syringe after the gas warms up?
25.8 mL.
Why must the container holding the gas be flexible in Charles' Law experiments?
A flexible container allows the gas to expand as it warms, preventing pressure buildup.
What would happen if a gas in a non-flexible container is heated?
The volume would increase until the pressure causes the container to fail, potentially resulting in an explosion.
Why is the scenario with a non-flexible container not an illustration of Charles' Law?
Because Charles' Law holds pressure constant, and in this case, the pressure changes.
Name two examples of flexible containers for gases.
Ziptop bag and balloon.
What is the approximate temperature at which gases have a volume of 0 L?
Approximately -273°C, near absolute zero.
Why would gases have zero volume at absolute zero?
There would be zero molecular motion because there would be zero energy.
What is the formula representing the relationship between pressure and temperature when volume is constant?
P1/T1 = P2/T2.
What happens to the pressure of a gas in a rigid container when its temperature increases?
The pressure increases due to the increased kinetic energy of the gas molecules.
Give a real-world example that demonstrates the relationship between pressure and temperature in gases.
Aerosol cans should not be put in a fire because the pressure will increase as the temperature increases.
What is the Combined Gas Law?
P1V1/T1 = P2V2/T2.
What are the initial conditions of a weather balloon at sea level?
P1 = 1.00 atm, V1 = 5.04 L, T1 = 31°C (304.15 K).
What are the conditions of the weather balloon after it rises 500 ft?
V2 = 5.30 L, T2 = 14°C (287.15 K).
How do you calculate the pressure at a higher altitude using the Combined Gas Law?
Use the formula P1V1/T1 = P2V2/T2 to solve for P2.
What is the significance of Gay-Lussac's Law?
It describes how pressure and temperature are directly related when volume is held constant.
What happens to gas molecules when the temperature increases in a rigid container?
The molecules strike the walls with more force, resulting in greater pressure.
What is the relationship between temperature and molecular motion in gases?
Increased temperature results in increased molecular motion.
What is the importance of understanding gas laws in real-world applications?
Gas laws help predict how gases behave under different conditions, which is crucial in fields like meteorology and engineering.
What happens to the volume of a gas when it is cooled?
According to Charles' Law, the volume decreases as the temperature decreases.
What causes atmospheric pressure?
Atmospheric pressure is caused by the collisions of air particles.
How does altitude affect air density and atmospheric pressure?
At high altitudes, air density is low, leading to fewer collisions and lower atmospheric pressure.
What is a barometer?
A barometer is an instrument used to measure atmospheric pressure.
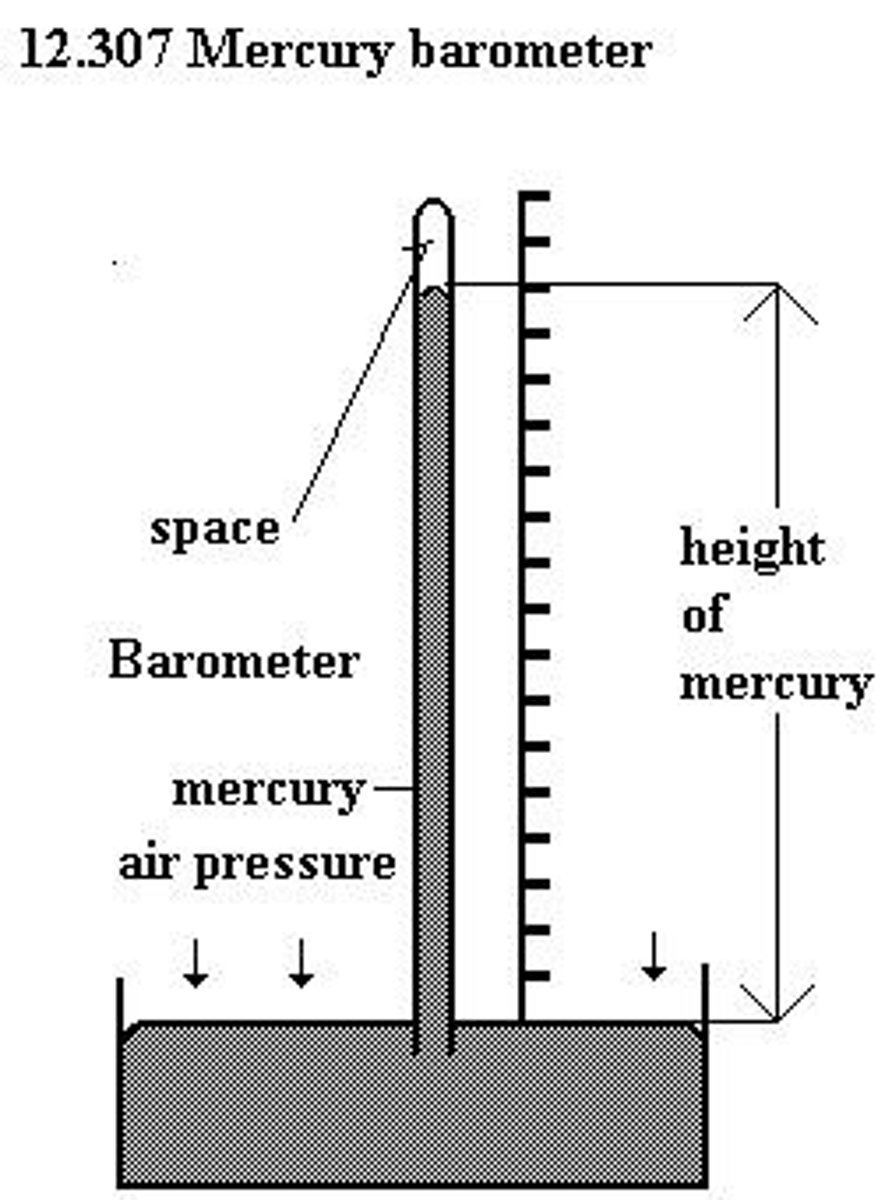
Who discovered the first barometer?
The first barometer was discovered by Evangelista Torricelli.
What is standard atmospheric pressure in mmHg?
Standard atmospheric pressure is 760 mmHg.
What does mmHg stand for?
mmHg stands for millimeters of mercury.
How is 1 atm defined in terms of mmHg?
1 atm is defined as 760 mmHg.
How would a barometer appear at Mile High Stadium in Denver?
The height of the mercury would be less than 760 mm because there is less atmospheric pressure pushing on it.
Why is mercury used in barometers instead of water?
Mercury is used because it is denser than water; a column of water would need to be impractically tall to measure the same pressure.
What is the density of water and mercury?
The density of water (DH2O) is 1.0 g/mL, while the density of mercury (DHg) is 13.6 g/mL.
How does a tire-pressure gauge work?
A tire-pressure gauge measures the pressure of air in a tire, typically using a spring mechanism that moves a needle on a dial.
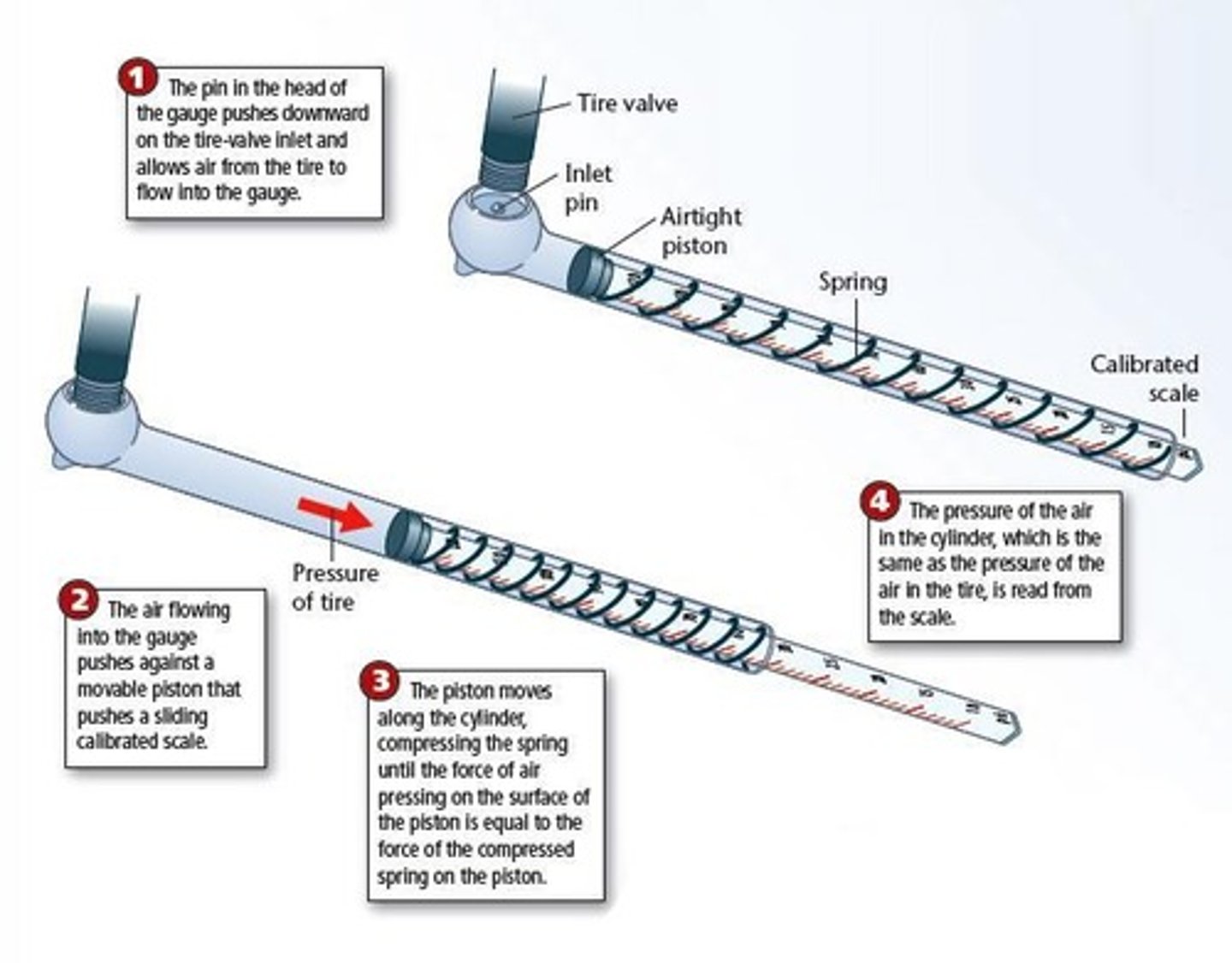
What are some common units used to measure pressure?
Common units include atm, mmHg, torr, inHg, Pa, kPa, and psi.
What is the atmospheric pressure in inches Hg on a sunny day in Cincinnati?
The atmospheric pressure might be around 30.45 inHg.
How do you convert inches Hg to atm?
To convert inches Hg to atm, use the conversion: 30.45 inHg × (1 atm / 29.9 inHg) = 1.018 or 1.02 atm.
What does a 'falling barometer' indicate?
A 'falling barometer' indicates that atmospheric pressure is dropping suddenly, often associated with stormy weather.
What is the atmospheric pressure in inches Hg during a storm in Cincinnati?
The atmospheric pressure might drop to 29.15 inHg.
How do you convert 29.15 inHg to mmHg?
To convert, use the formula: 29.15 inHg × (760 mmHg / 29.9 inHg) = 740.936 mmHg, rounded to 741 mmHg.
Where can you find the optimum tire pressure for a car?
The optimum tire pressure can be found in the car manual, on the driver's side door frame, or on the tire itself.
How do you convert tire pressure from psi to kPa?
To convert psi to kPa, use the conversion factor: 1 psi = 6.89476 kPa.
What is the relationship between pressure and density in the context of atmospheric pressure?
Higher density of air at lower altitudes results in greater atmospheric pressure due to more frequent collisions of air particles.
What is the significance of standard atmospheric pressure?
Standard atmospheric pressure is a reference point for various scientific calculations and measurements.
What happens to atmospheric pressure as altitude increases?
As altitude increases, atmospheric pressure decreases due to lower air density.
What is the atmospheric pressure at sea level in kPa?
101.325 kPa.
What is the atmospheric pressure at Wheeler Peak compared to sea level?
It is lower than 101.325 kPa.
Why is it easier to drink soda through a straw in New Orleans than at Wheeler Peak?
Because New Orleans has greater air pressure, which pushes the liquid up the straw more effectively.
What happens to the air pressure when Anthony sips his soda?
The pressure in his mouth decreases, allowing air pressure to push the liquid up the straw.
What does Dalton's Law of Partial Pressures state?
The total pressure is equal to the sum of the pressures of all the gases in a mixture.
How is partial pressure defined?
The pressure of a single gas in a mixture as if that gas alone occupied the container.
What is the formula for total pressure according to Dalton's Law?
P total = P1 + P2 + P3 + .......Pn.
What assumption does Dalton's Law rely on regarding gas molecules?
Gas molecules act independently of one another in a mixture.
Under what conditions does Dalton's Law hold true?
It is most accurate for ideal gases and when there is a lot of space between gas molecules.
What effect does lowering the temperature have on gas behavior?
It can upset the assumption that gas molecules act independently.
Why might Dalton's Law be valuable in a chemistry lab?
It helps in calculating the pressures of individual gases in mixtures.
What is the significance of the barometer readings in the context of Wheeler Peak and New Orleans?
Barometer B shows lower pressure at Wheeler Peak, while Barometer A shows higher pressure at sea level in New Orleans.