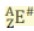Ch 2 - Atoms, Ions, & Molecules
0.0(0)
Card Sorting
1/8
Earn XP
Description and Tags
Last updated 9:17 PM on 10/16/22
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
9 Terms
1
New cards
Law of Conservation Mass
Mass is neither created nor destroyed on a chemical reaction. The mass before and after a chemical reaction does not change
2
New cards
Law of Definite Proportions/ Law of Constant Composition
All samples of a compound have the same composition - the same proportions by mass of the constituent elements
3
New cards
Law of Multiple Proportions
If two elements form more than a single compound, the masses of one element combined with a fixed mass of a second are in the ratio of small, whole number
4
New cards
E is the ...
Elemental symbol
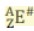
5
New cards
Z is the ...
Atomic number and equals the number of protons
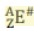
6
New cards
A is the ...
Mass number and equals the number of protons and neutrons
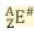
7
New cards
A - Z equals...
Number of neutrons
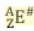
8
New cards
# is the ...
charge (can be positive, negative, or zero)
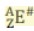
9
New cards
Z - # equals ...
Number of electrons