Chap 15B - Arenes (Benzene)
1/11
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
12 Terms
State reagents and conditions for halogenation
Cl2, anhydrous AlCl3 catalyst (or FeCl3 catalyst)
Br2, anhydrous AlBr3 catalyst (or FeBr3 catalyst)
Fe can also be used as it is converted to iron(III) halide catalyst in situ (reaction mixture) by reaction with the halogen reagent 2Fe + 3X2 → 2FeX3
The Lewis acid catalyst, AlCl3 or AlBr3 (or FeCl3 or FeBr3), must be dry (anhydrous) to prevent hydrolysis from taking place -> so it cannot act as a Lewis acid to generate the strong electrophile
The Al atom in AlCl3 or AlBr3 is electron deficient as there are only six valence electrons around it
Al in AlCl3 and AlBr3 have energetically accessible vacant orbitals to accept a lone pair of electrons -> able to act as Lewis acids
Describe observations of halogenation
When Cl2 is used, yellowish-green Cl2 is decolorized and white fumes of HCl are formed
When Br2 is used, orange-red Br2 is decolorized and white fumes of HBr are formed
Under prolonged conditions and in the presence of a higher proportion of the halogen, the 1,2-dihalide and 1,4-dihalide are formed
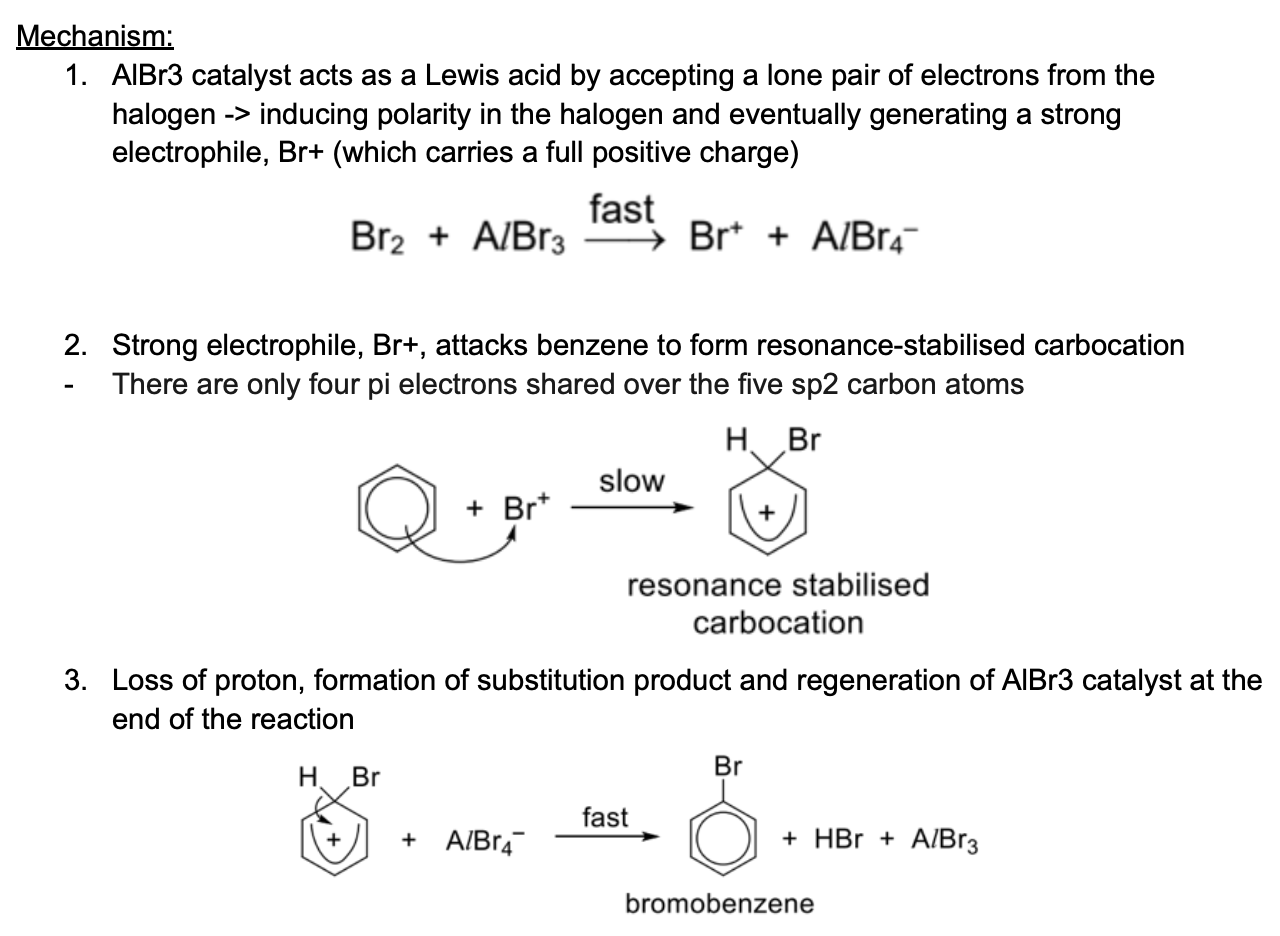
Describe mechanism of halogenation
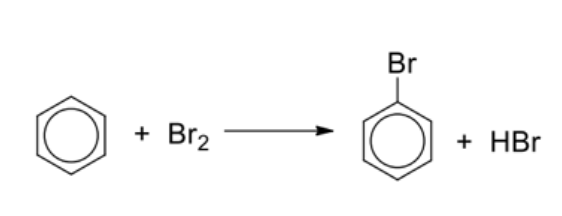
Describe general reaction of halogenation
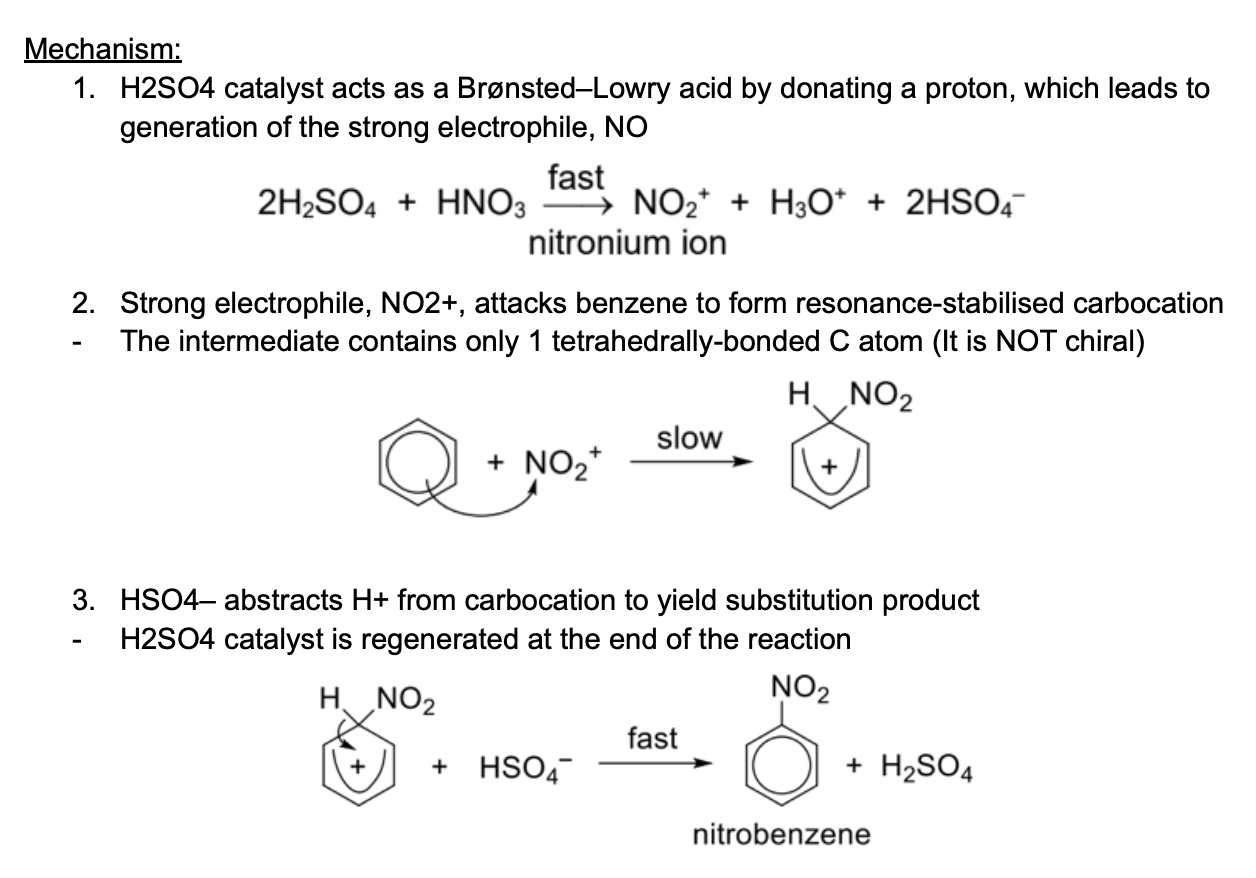
Describe reagents and conditions of nitration
Reagents and conditions: concentrated HNO3, concentrated H2SO4 catalyst, heat at 55-60C (under reflux)
Nitrobenzene is a pale yellow liquid with an almond smell
Temperature for this reaction needs to be carefully controlled as high temperatures (100 C or above) will lead to formation of the di-substituted product or tri-substituted product
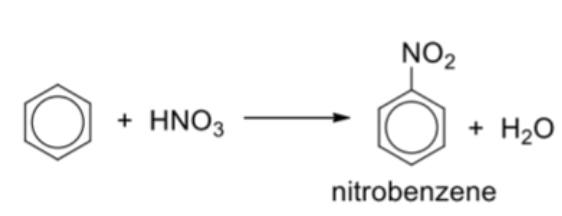
Describe general reaction of nitration
Describe reflux apparatus
|
Describe nitration mechanism
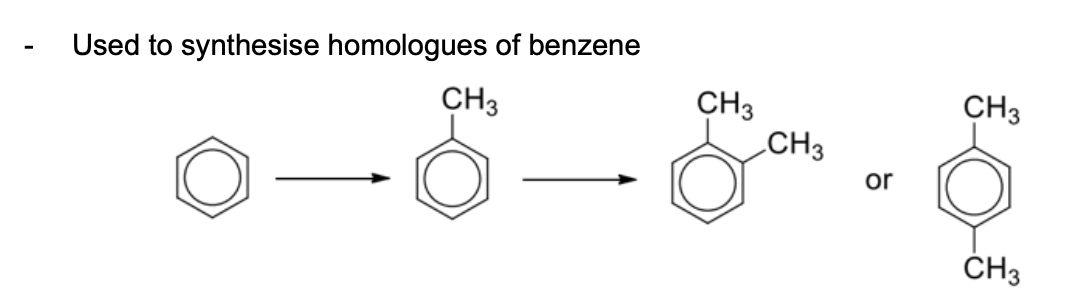
Describe reagents and conditions of friedel-crafts alkylation
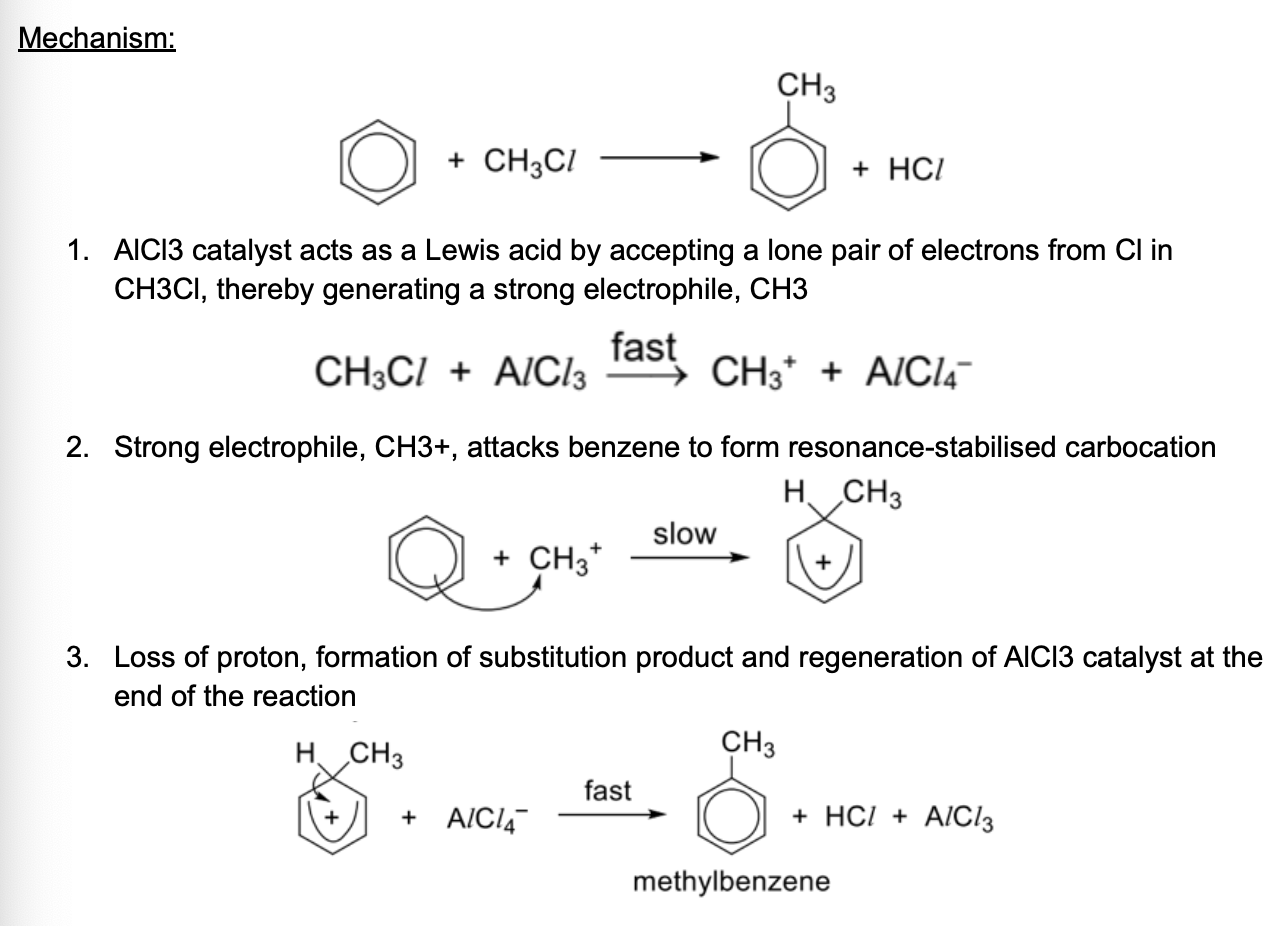
RCl, anhydrous AlCl3 catalyst (or FeCl3 catalyst), excess benzene
RBr, anhydrous AlBr3 catalyst (or FeBr3 catalyst), excess benzene
Al in AlCl3 and AlBr3 have energetically accessible vacant orbitals and are electron-deficient molecules to accept a lone pair of electrons
AlCl3 and AlBr3 must be anhydrous to prevent hydrolysis from taking place
Necessary to start with excess benzene to prevent further alkylation which occurs even more readily
Used to synthesise homologues of benzene (multi-substituted benzene)
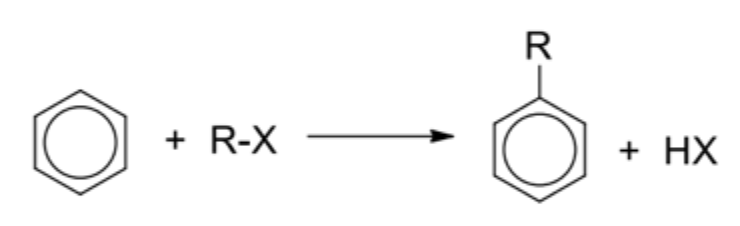
Describe general reaction of friedel-crafts alkylation
Describe mechanism of friedel-crafts alkylation
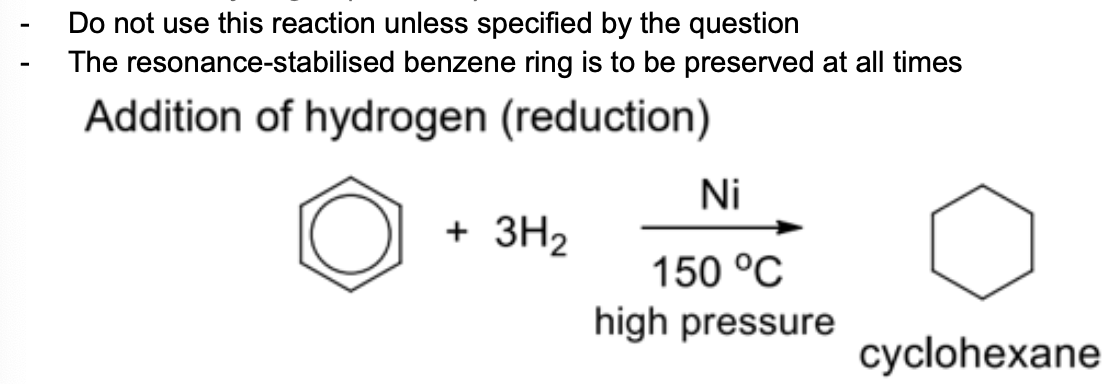
Describe addition of hydrogen (reduction) +.general equation