Subatomic Particles
1/23
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
24 Terms
Atom
The smallest particle of an element that retains the properties of that element; is electrically neutral, spherical in shape, and composed of protons, neutrons, and electrons
Atomic mass (average atomic mass)
The weighted average mass of the isotopes of that element
Atomic Mass Unit (amu)
One twelfth the mass of a carbon-12 atom
Atomic Number
The number of protons in the nucleus of an atom
Electron
A negatively charged, fast-moving particle with an extremely small mass that is found in all forms of matter and moves through the empty space surrounding an atom's nucleus (has charge of -1)
Isotope
Atoms of the same element with the same number of protons but DIFFERENT numbers of neutrons
Mass number
The number after an element's name, representing the sum of its protons and neutrons
Neutron
A neutral, subatomic particle in an atom's nucleus that has a mass nearly equal to that of a proton
Nucleus
The extremely small, positively charged, dense center of an atom that contains positively charged protons and neutral neutrons
Proton
A subatomic particle in an atom's nucleus that has a positive charge of 1+
Ion
An atom or group of atoms that has a positive or negative charge.
Anion
Negatively charged ion (gained electrons)
Cation
Positively charged ion (lost electrons)
Nuclide symbol
Symbol for a nuclide in which the mass number is written as a superscript and the atomic number as a subscript on the left of the symbol for the element
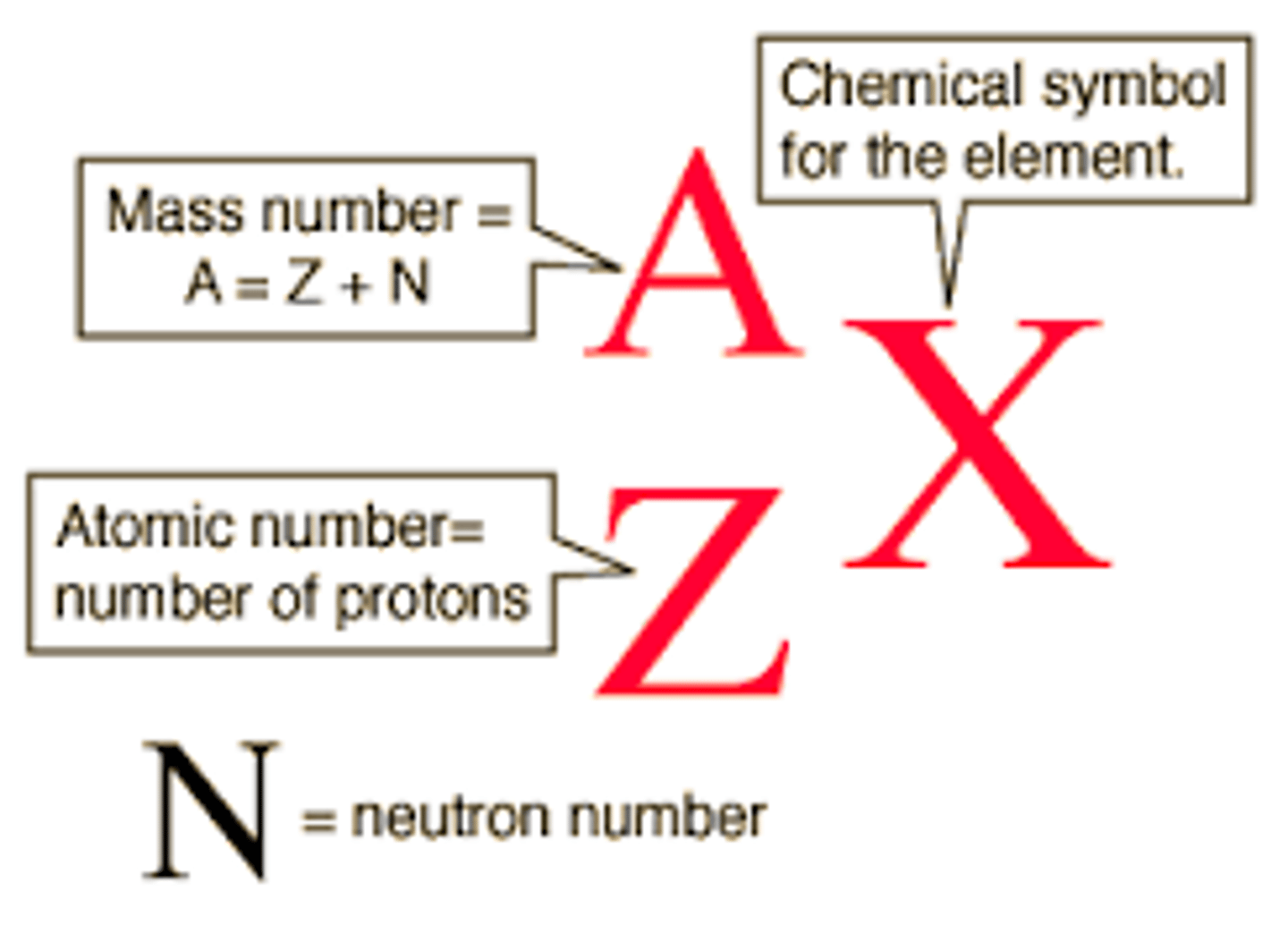
Democratis
First to discover/ think of the atom, said that all matter consists of very small, indivisible particles called atoms
John Dalton's Atomic Theory
1. elements are made from tiny particles called atoms. 2. all atoms of a given element are identical 3.atoms cannot be subdivided, created or destroyed. 4. atoms combine in simple whole number ratios to form compounds 5. in chemical reactions, atoms are rearranged
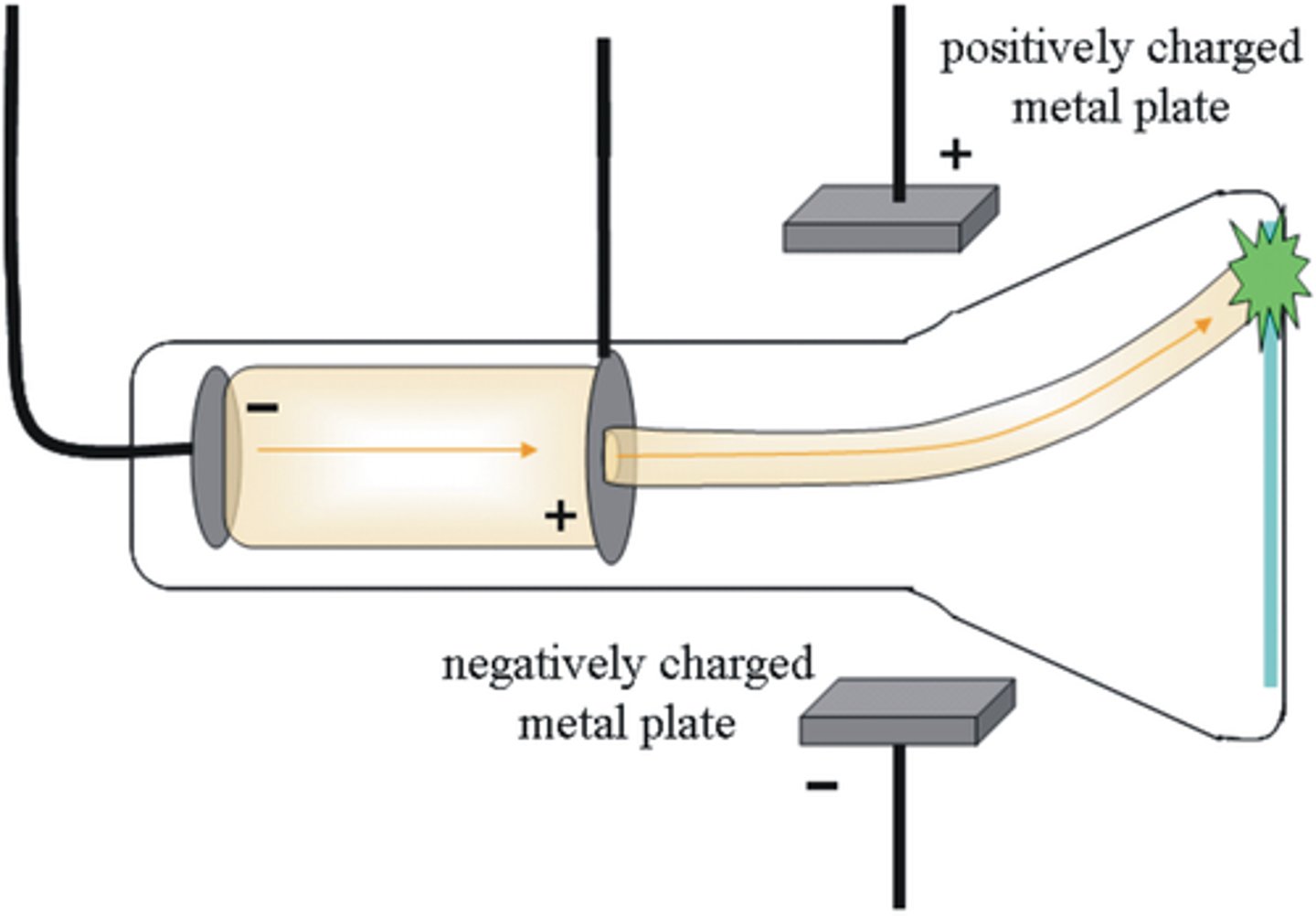
JJ Thomson
Used the cathode ray tube to discover electrons
James Chadwick
Discovered the neutron
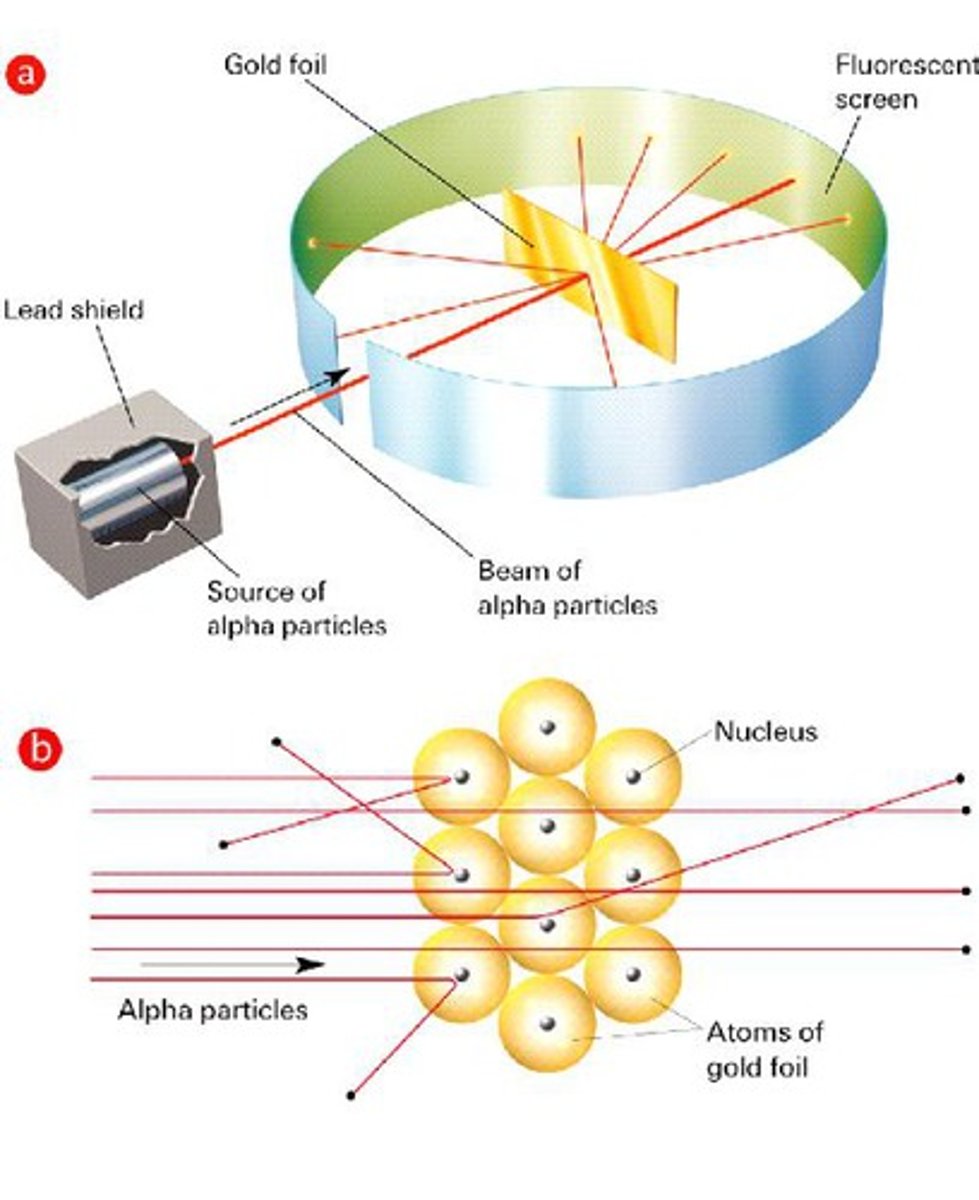
Ernest Rutherford
Gold foil experiment, discovered nucleus
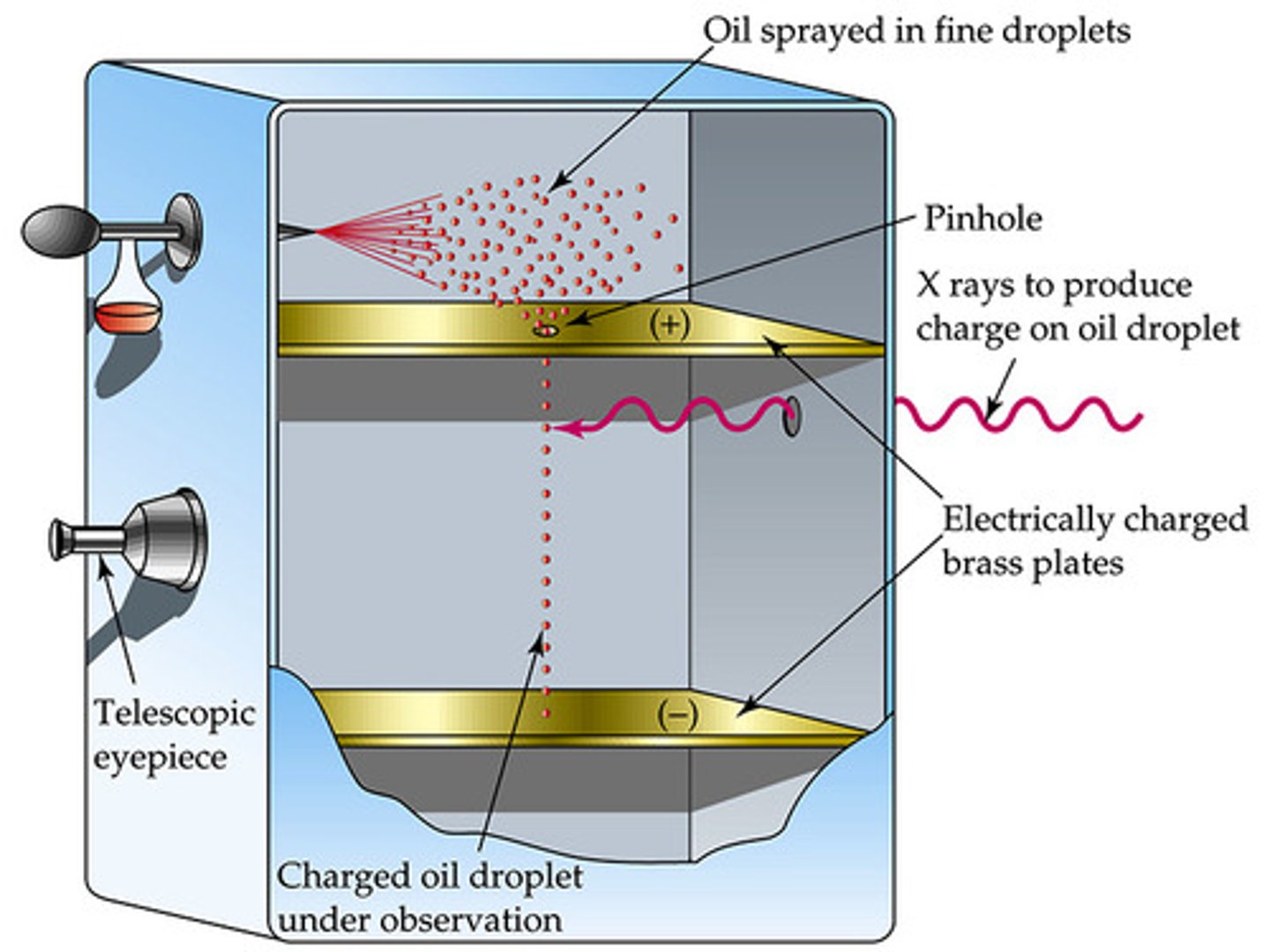
Robert Millikan
1909 oil drop experiment, calculated the charge of the electron, calculate the mass of electrons
Henry Moseley
Found that all atoms of a given element contained the same number of protons in the nucleus
Cathode Ray Experiment
An experiment that showed that electrons had mass and a charge
Gold Foil Experiment
Conducted by Ernest Rutherford in which alpha particles that were shot at gold foil were deflected when they hit the positive center of gold atoms. The nucleus was discovered as a result of this experiment.
Oil Drop Experiment
A drop of oil was placed in a cylinder with negative and positive plates, the drops were charged using an x-ray. Different waves of xrays let the drops fall at different rates. Determined that the mass of the electron is 9.10 x 10^-28 by Robert Milikan