chem exam 1
1/214
Earn XP
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
215 Terms
matter
has mass & takes up space
substance
specific type of matter
states
solid, liquid, gas
scales
atomic (small) & macroscopic (large)
solid
close together & ordered arrangement of atoms, strong
liquid
disordered arrangement, but interacting atoms, some strength
gas
far apart and no interaction of atoms, no strength
energy
-capability to do work
-moving an object against an opposing force
-can be kinetic or potential energy
energy of motion
more motion = more energy
temperature
measure of average KE of particles. higher temp, higher KE
heat
moves from high to low
potential energy
- stored energy, through position or composition
- the more unstable, the higher the PE
-reactions go towards the most stable state
difference
heat energy released when fuel (high PE) is burned
PE & food
- carbs & fats, high PE
- products of metabolism low PE
- energy is released to power metabolism
metric system
used in medicine and science
SI system
international unit
meter (m)
distance, length
gram (g)
mass, weight
liter (L)
volume
second (s)
time
1mL
1cm³
sig figs
- “guess” one place value beyond what can be read
- record all certain digits, assume uncertain
placeholder zeros
-aren’t significant!
-zeros in a decimal before the first integer
- large numbers with many zeros but no decimals
exact numbers
- obtained by counting
- whole numbers
- defined quantities
- no uncertainty or sig figs
round to
- the smallest amount of sig figs in an equation
- at the end!
conversion factors
1. identify given & asked for units
2. identify conversion between given & asked
3. express conversion a two conversion factors
4. multiply given by conversion factor
5. solve & round
density
- d = m/V
- units: g/mL, g/cm³
specific gravity
- density of substance/density of water!!
- important for diagnosis kidney function conditions (low-over hydrated, high-dehydrated)
- density of water is 1.000g/mL
normal specific gravity
1.002-1.030
dosage
mg drug/kg body weight
q.d
once a day
q.i.d
4x a day
b.i.d
2x a day
fahrenheit
- relative, uses + & -
- US only
- NOT for medicine
celsius
- worldwide!
- relative, uses + & -
normal body temp
37C, 98F
boiling temp
100C, 212F
freezing temp
0C, 32F
conversion between C &F
-C = (F-32)/1.8
-F = 1.8C + 32
atoms
- sphere
- protons & neutrons = nucleus
- electrons surround nucleus, fast & random
proton
positive
neutron
neutral
electron
negative
atom mass
- from protons and neutrons
- electrons have negligible mass, determine volume
charge
protons & electons
atomic number
number of protons
atomic mass
avg mass of isotopes of the same element
atomic symbol
uppercase letter then lowercase letter

isotopes
- same protons & electrons, diff neutrons
- identified by mass #
isotope nuclear symbol
- mass # superscript
- atomic # subscript

groups of elements
exhibit similar properties
groups 1A-8A
main!
1A
alkali metals
2A
alkaline earth metals
7A
halogens
8A
noble gasses (most stable)
groups 1B-8B
-transition metals
-inner transition metals
periods
rows
metals, nonmetals, metalloids
- bold diagonal steps separate left metals from nonmetals on right
-Al is a metal!
metals
- exist as a solid at room temp, except Hg
- shiny
- good conductors
- flexible
nonmetals
- dull
- exist as solids, liquids, & gasses
- poor conductors
metalloids
properties in between metals and nonmetals
electron arrangement
determines physical and chemical properties
quantum mechanics
- electrons have certain allowed energy levels
- n = symbol for levels
- the larger n is, the smaller the difference between energy levels is
- n = 2n²
2 valence electrons
level 1
8 valence electrons
levels 2 & 3
valence electrons
- electrons in the outermost shell
- does not equal total electrons
periodicity
repeating patterns found in the periodic table
octet rule
- 8 is very stable
- electrons want a filled valence shell
ionic compounds
- salts!
- charged ions
- form when metal atoms transfer electrons to nonmetal atoms
- held together by strong opposite charges
- net charge
-structure determines function
- compounds have different properties than their elements
cation
- from metal losing electrons
- positive charge in superscript
anion
- negative charge in superscript
- from nonmetals gaining electrons
ionic bond
strong electrostatic attraction between oppositely charged ions
ions
change in electrons
ionic compound ratio
reflected in subscript
electrolytes
-ions dissolved in water conduct electricity
- super important in the body
cations 1A, 2A, 3A
- lose all valence electrons
- name of cation, same as element
- electron arrangement of noble gas before it
- Be & B exception
anions 5A, 6A, 7A
- nonmetals
- gain electrons to fill shell
- same electron arrangement as noble gas after
- name is element name w/ -ide ending
naming ionic compounds
-cation name + anion name
-no need for subscripts
writing formula unit of a ionic compound
- list cation and anion symbol w/ no charges
- make sure sum of charges is 0
- simplify when possible!
4A transition metals
- don’t always lose all their valence electrons
- name w/ roman numeral
- some fixed ions, silver, zinc, cadmium
silver ion
Ag⁺
zinc ion
Zn²⁺
cadmium ion
Cd²⁺
2 ways to figure out transition metal charge
- crisscross method
- math
heavy metals
- mainly Pb (lead II & IV) & Hg (mercury I & II)
- Pb²⁺ & Hg₂²⁺ are poisonous
ionic compounds in products
- calamine lotion → zinc oxide & iron(III) oxide
- many toothpastes contain fluoride
polyatomic ions
- come from molecules
- contain 1+ covalent bond
- unequal amt of protons & electrons
- unique name, formula, 7 charge
- few cations
cation polyatomic ions
- NH₄⁺ ammonium
- H₃O⁺ hydronium
writing formula unit for polyatomic ions
- same as monoatomic
- can use parenthesis on polyatomic ion if subscript is needed
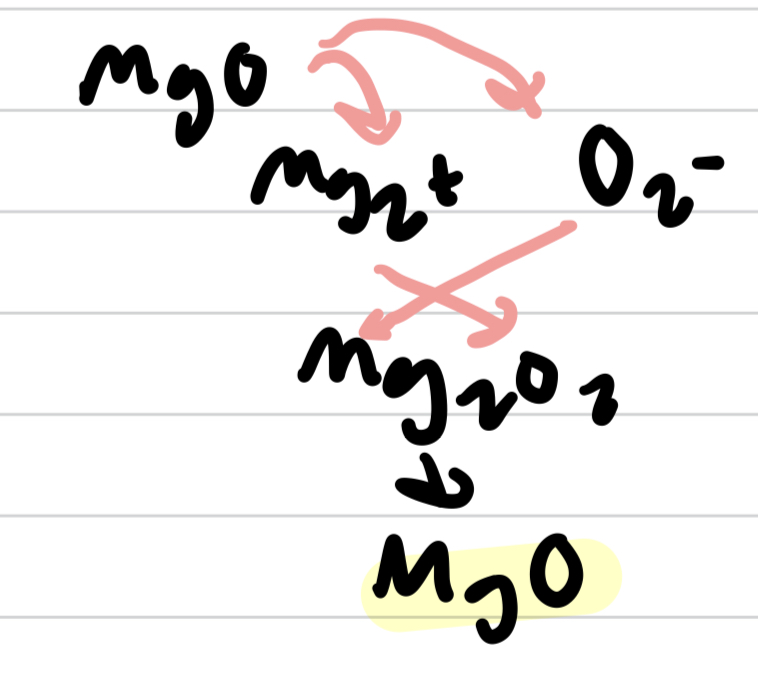
naming polyatomic ions
- name cation
- name anion (use name of polyatomic)
- be careful w/ suffixes!
polyatomic ions in products
- bleach
- preservatives
- tooth enamel
covalent compounds
- made of identical molecules, composed of 2+ nonmetal atoms joined by covalent bonds
- nonmetal ions joined
Si & B (metalloids) can too !
- includes many cellular compounds such as proteins, carbs, DNA, & RNA
- diff shapes & sizes
diatomic elements
- H₂, N₂, O₂, F₂, Cl₂, Br₂, I₂
(H7 on periodic table)
- never by themselves, always paired up w/ another element
the covalent bond
- 2 hydrogen atoms → H₂
- valence electrons shared
- has noble gas electron arrangement
- more stable atoms
molecular formulas
- covalent molecule
- specifies # of each type of atom in each molecule as subscripts, usually alphabetical
- differs from ionic formulas
- DON’T simplify subscripts
glucose C6H12O6
naming simple binary compounds
- name first element, then second w/ -ide
- insert prefixes indicating # of each atom type
- mono- only for second element
one
mono
two
di
three
tri