VSPER Shapes
1/29
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
30 Terms
VSEPR model
attempts to minimize electron pair repulsions in an atom which allows it to predict shape
2 electron pairs, 0 lone pairs
linear
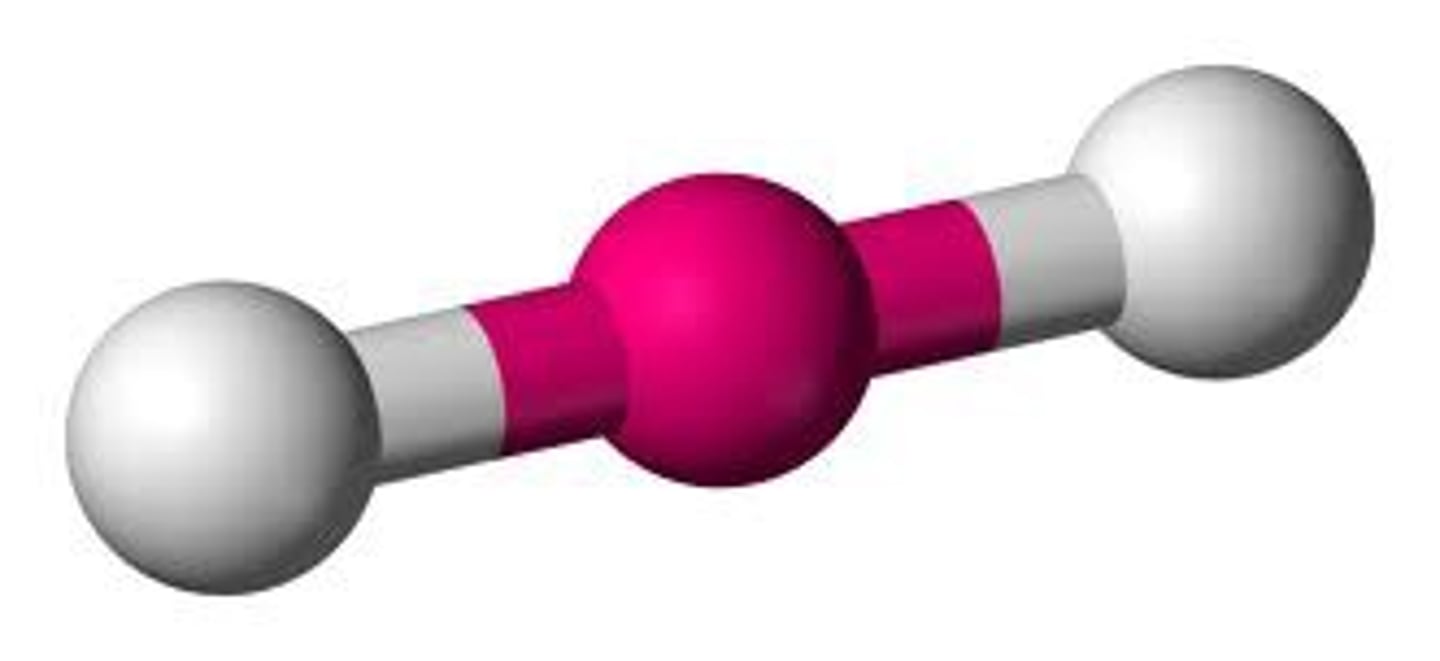
linear bond angle
180 degrees
3 electron pairs, 0 lone pairs
trigonal planar
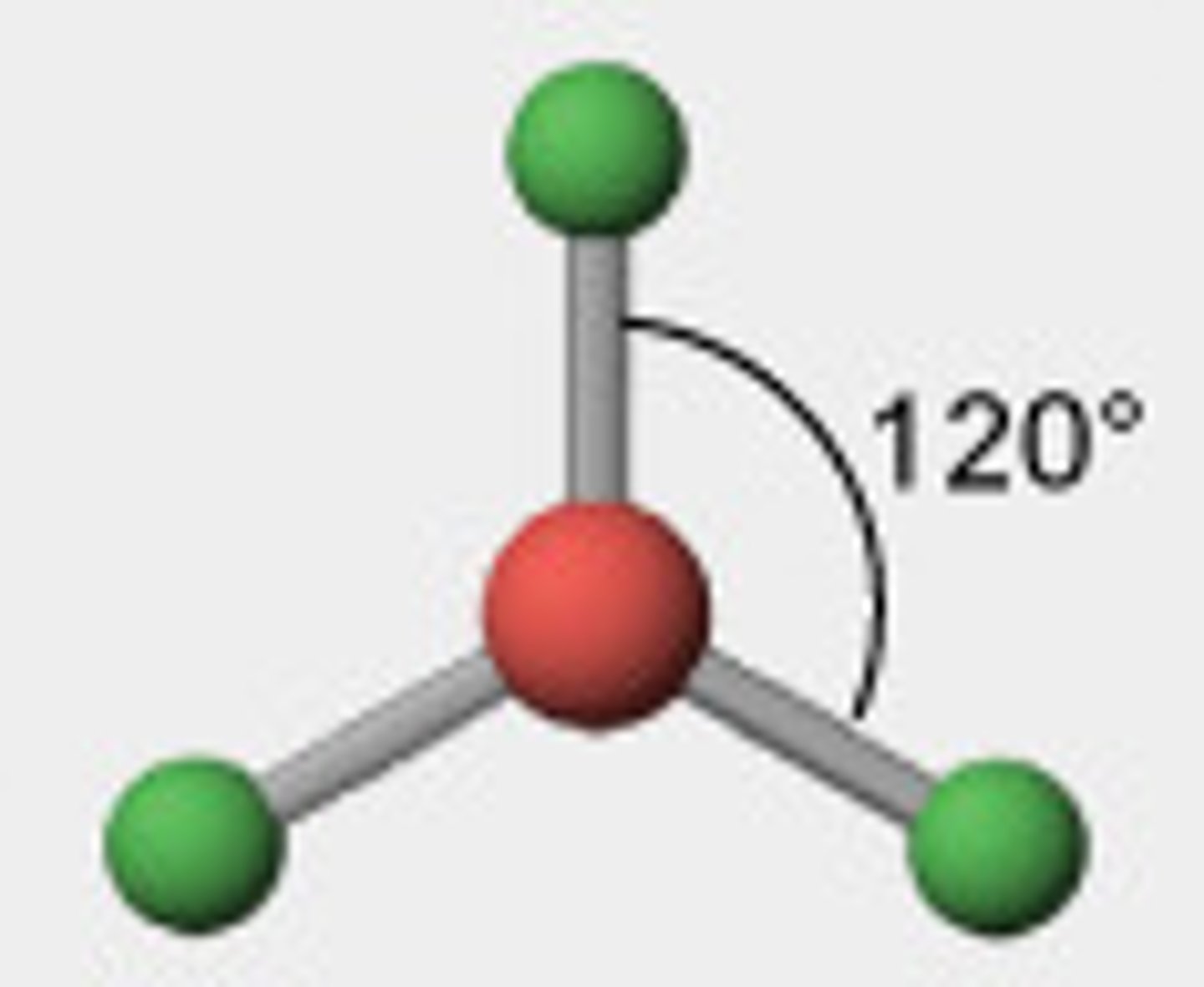
trigonal planar bond angle
120
3 electron pairs, 1 lone pair
bent
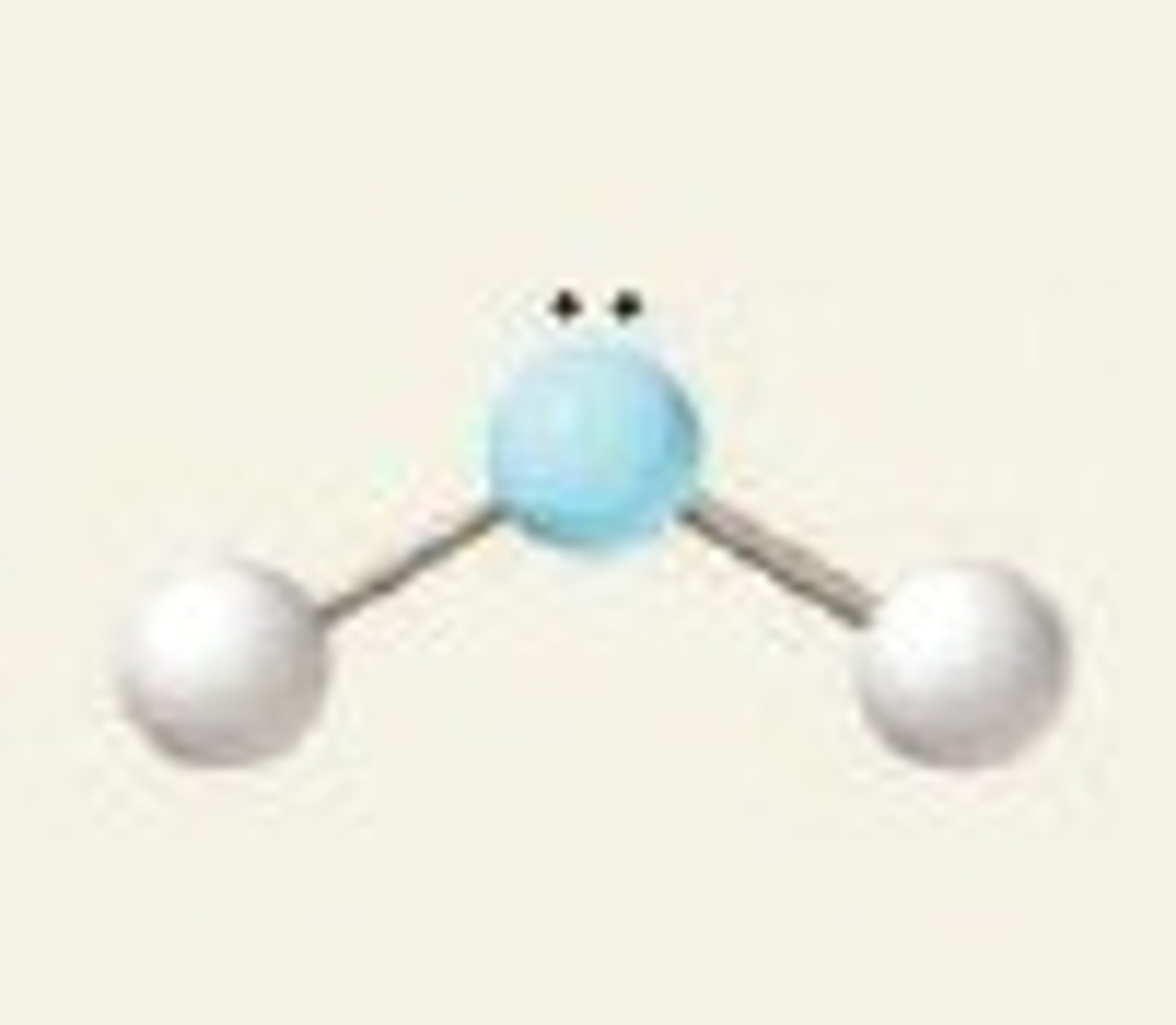
bent bond angle for 3 electron pairs, 1 lone pair
less than 120 degrees
4 electron pairs, 0 lone pair
tetrahedral
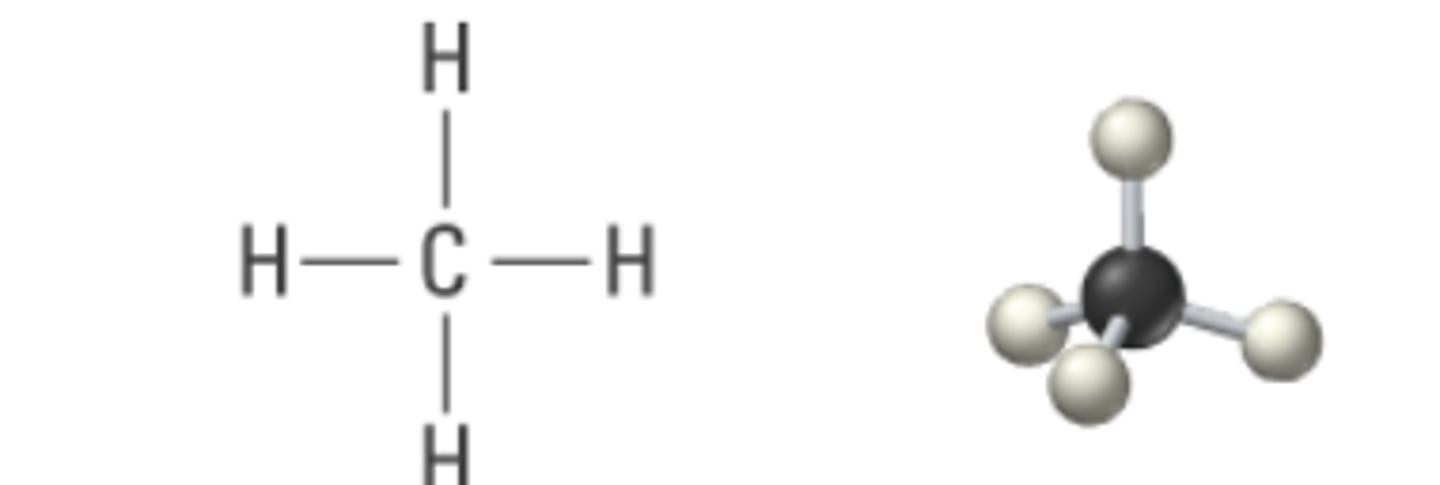
tetrahedral bond angle
109.5
4 electron pairs, 1 lone pair
trigonal pyramidal
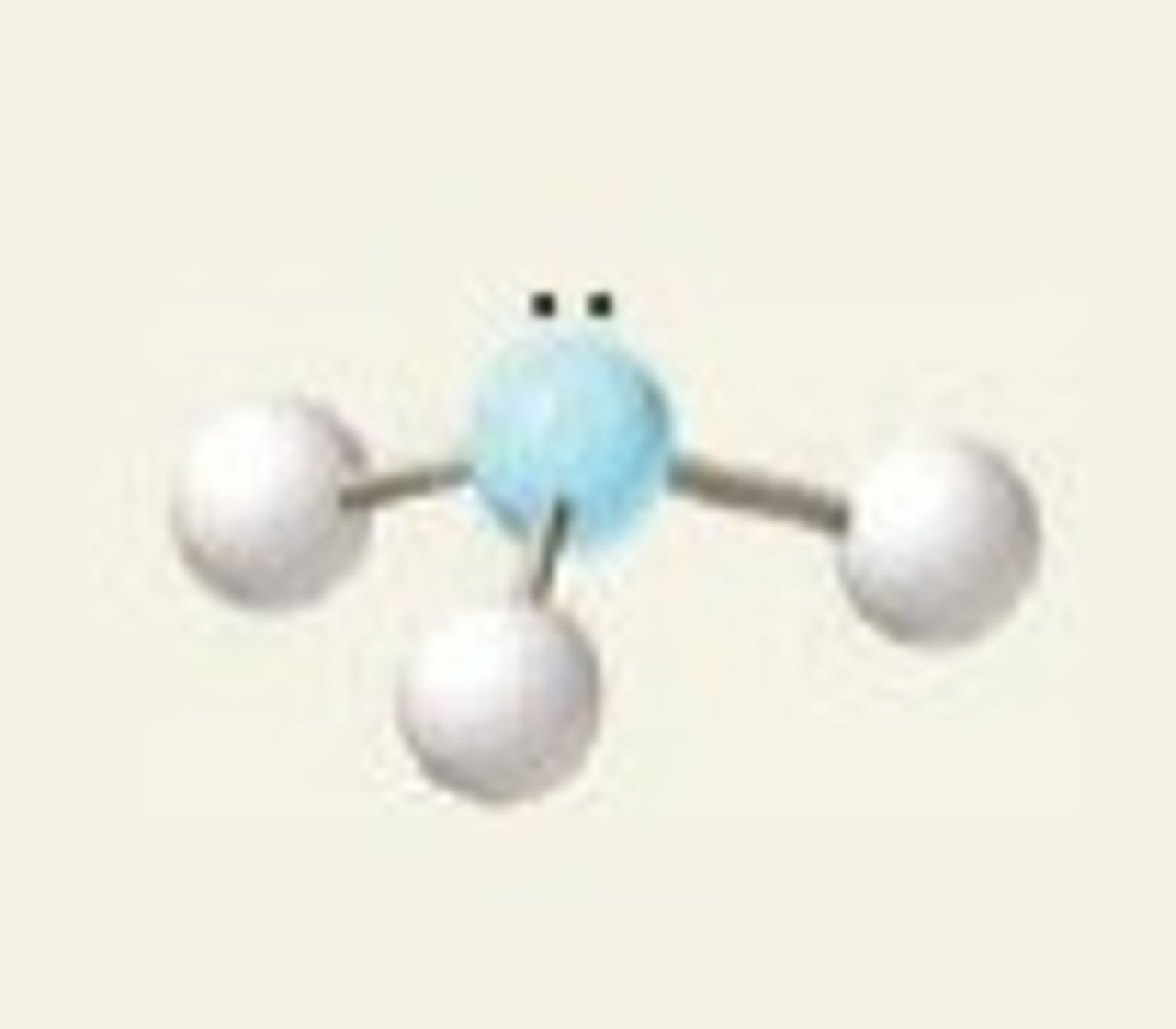
trigonal pyramidal angle
109.5 due to electron pair geometry
slightly less due to molecular geometry
4 electron pairs, 2 lone pairs
bent
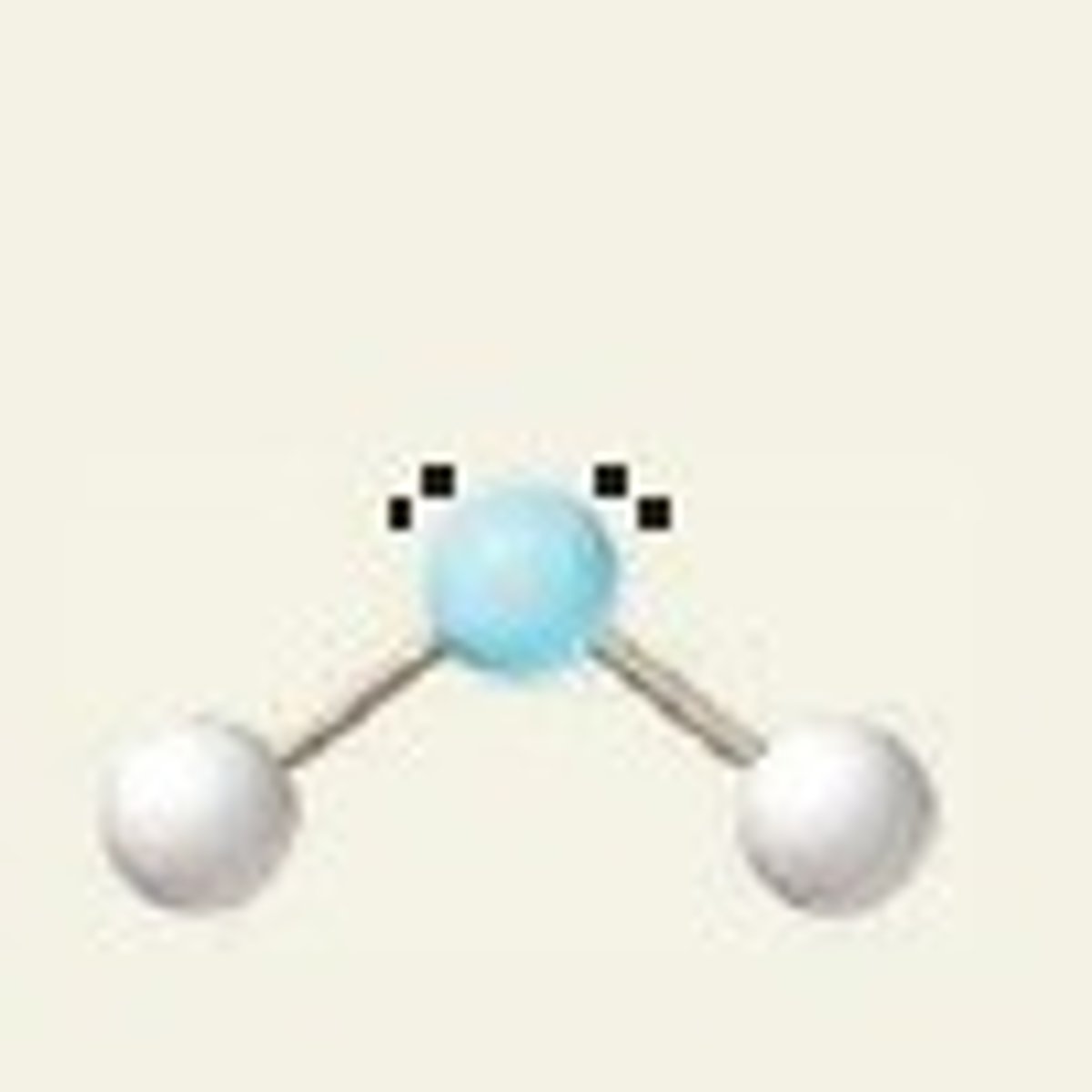
bent angle
109.5 due to electron pair geometry
actually 104 due to molecular geometry
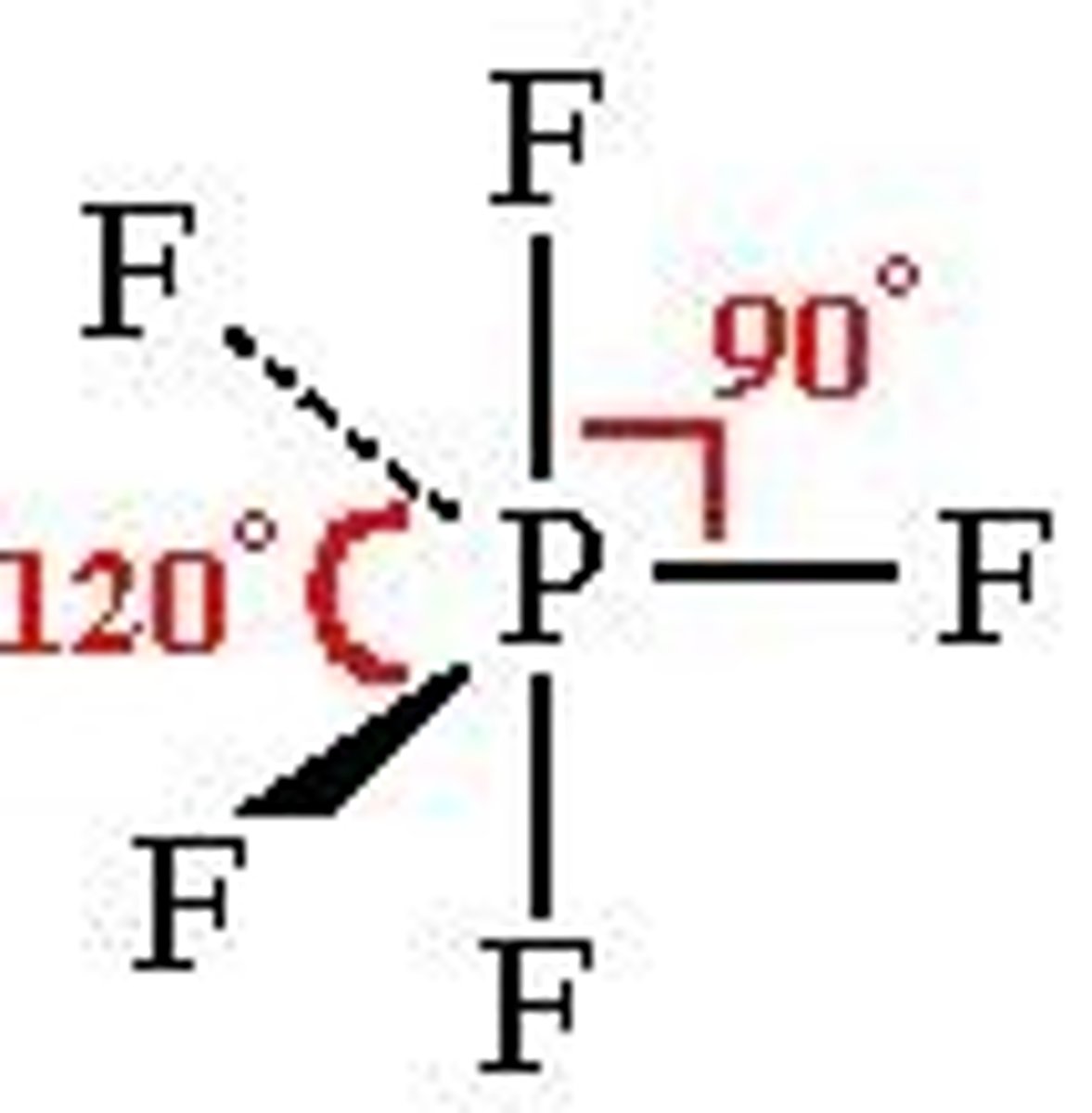
5 electron pairs, 0 lone pairs
trigonal bipyramidal
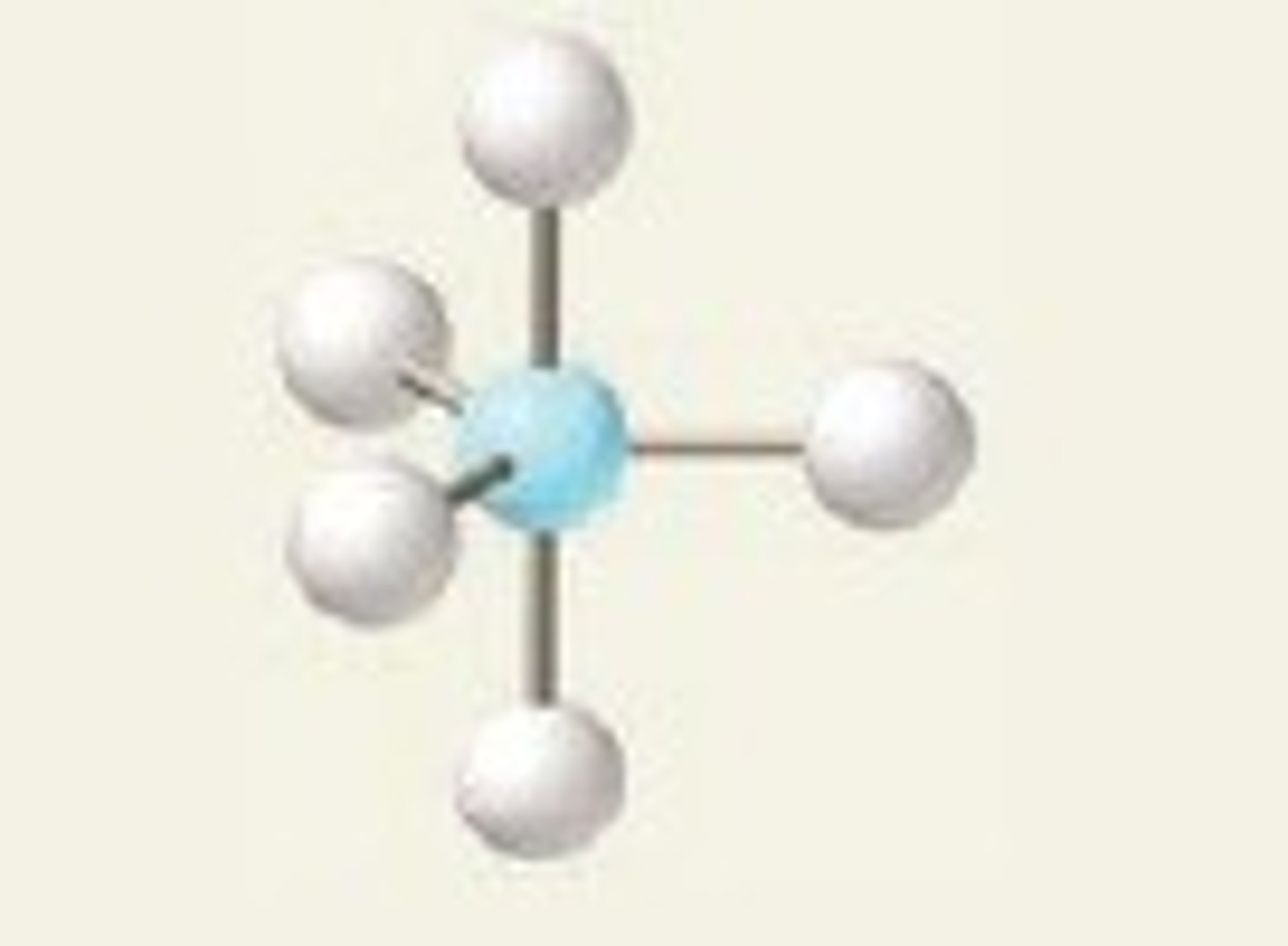
trigonal bipyramidal angle
90 for atoms on same plane
120 for atoms on different planes
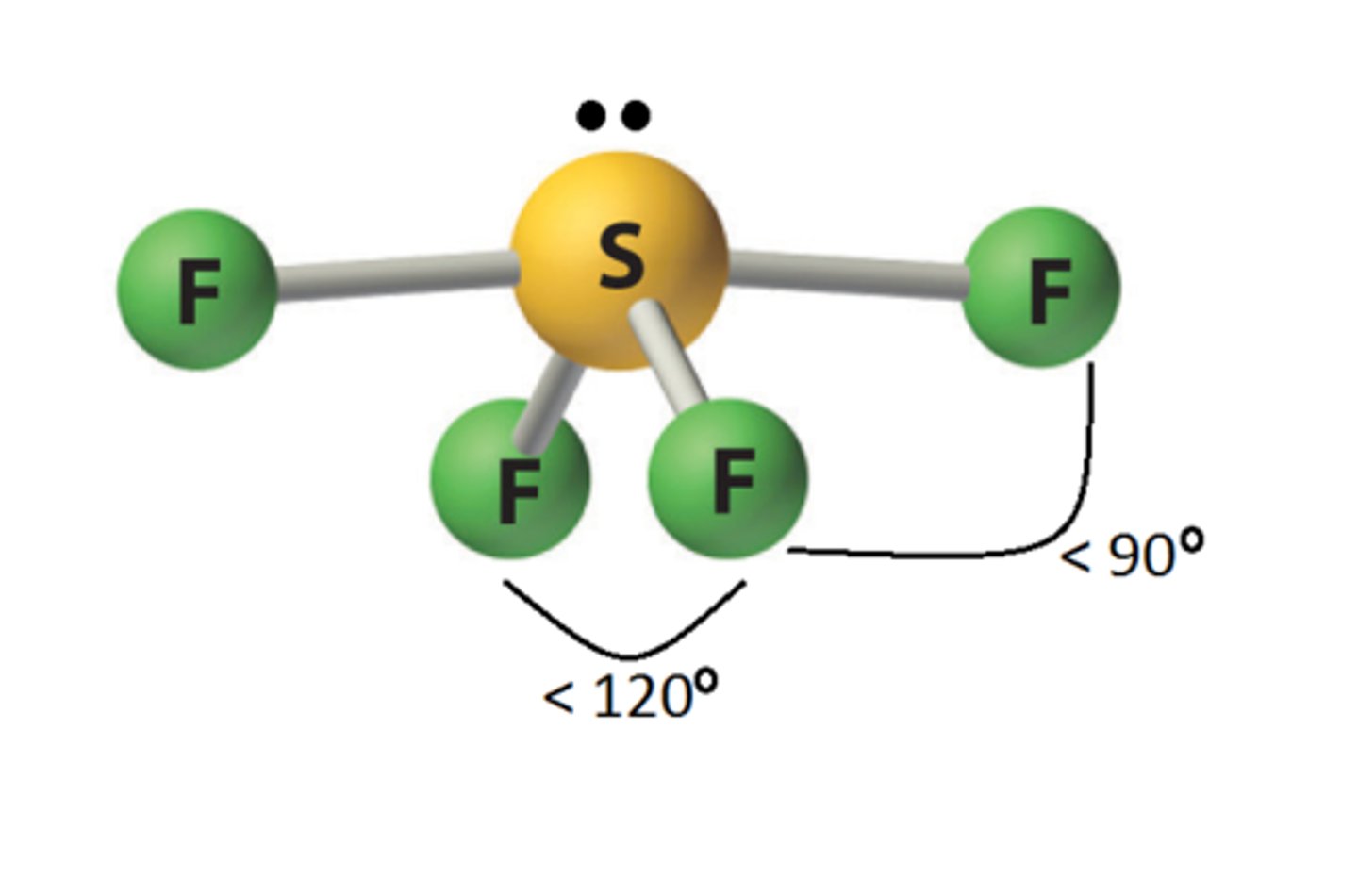
5 electron pairs, 1 lone pair
seesaw
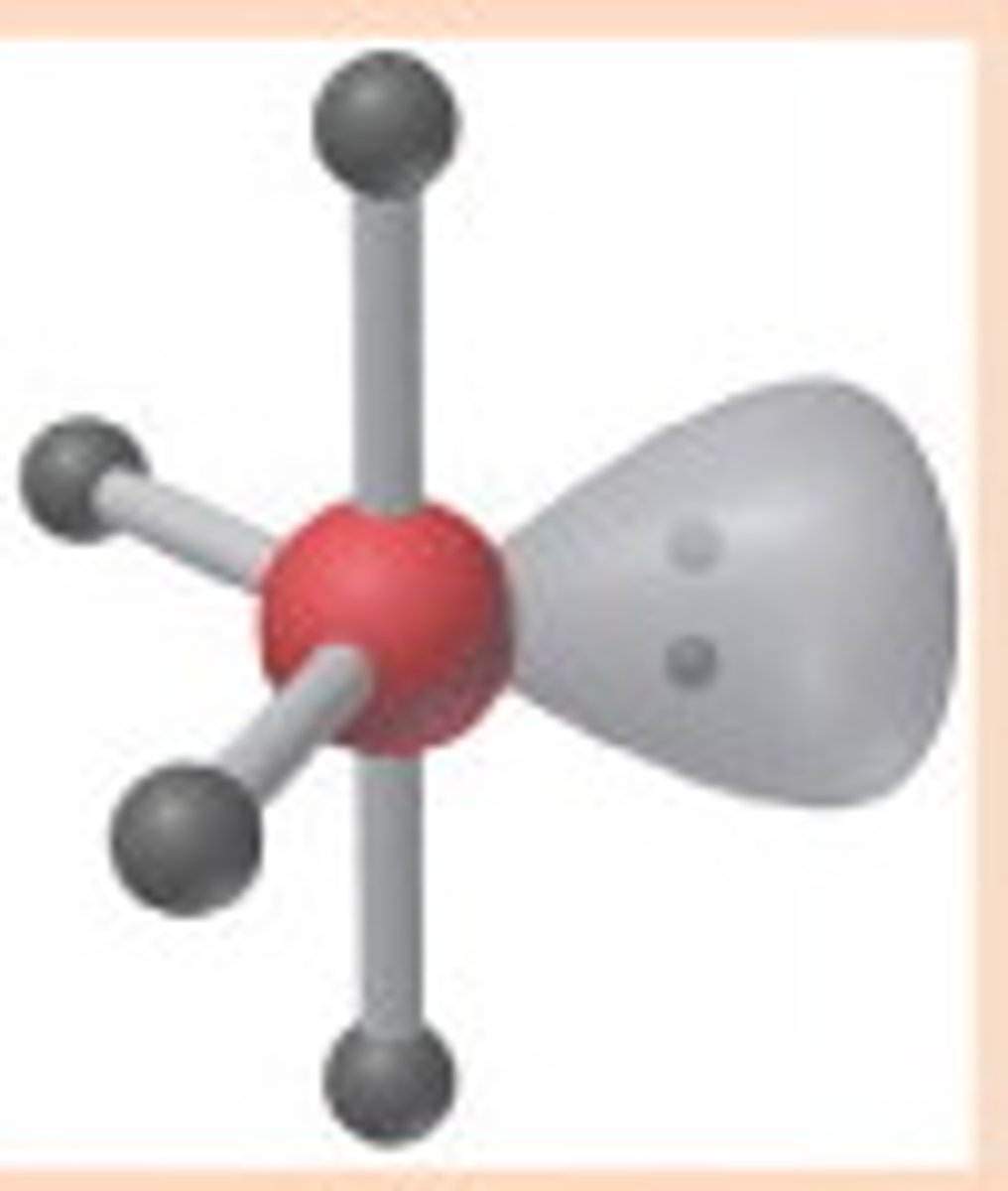
seesaw angle
90-120
5 electron pairs, 2 lone pairs
T shaped
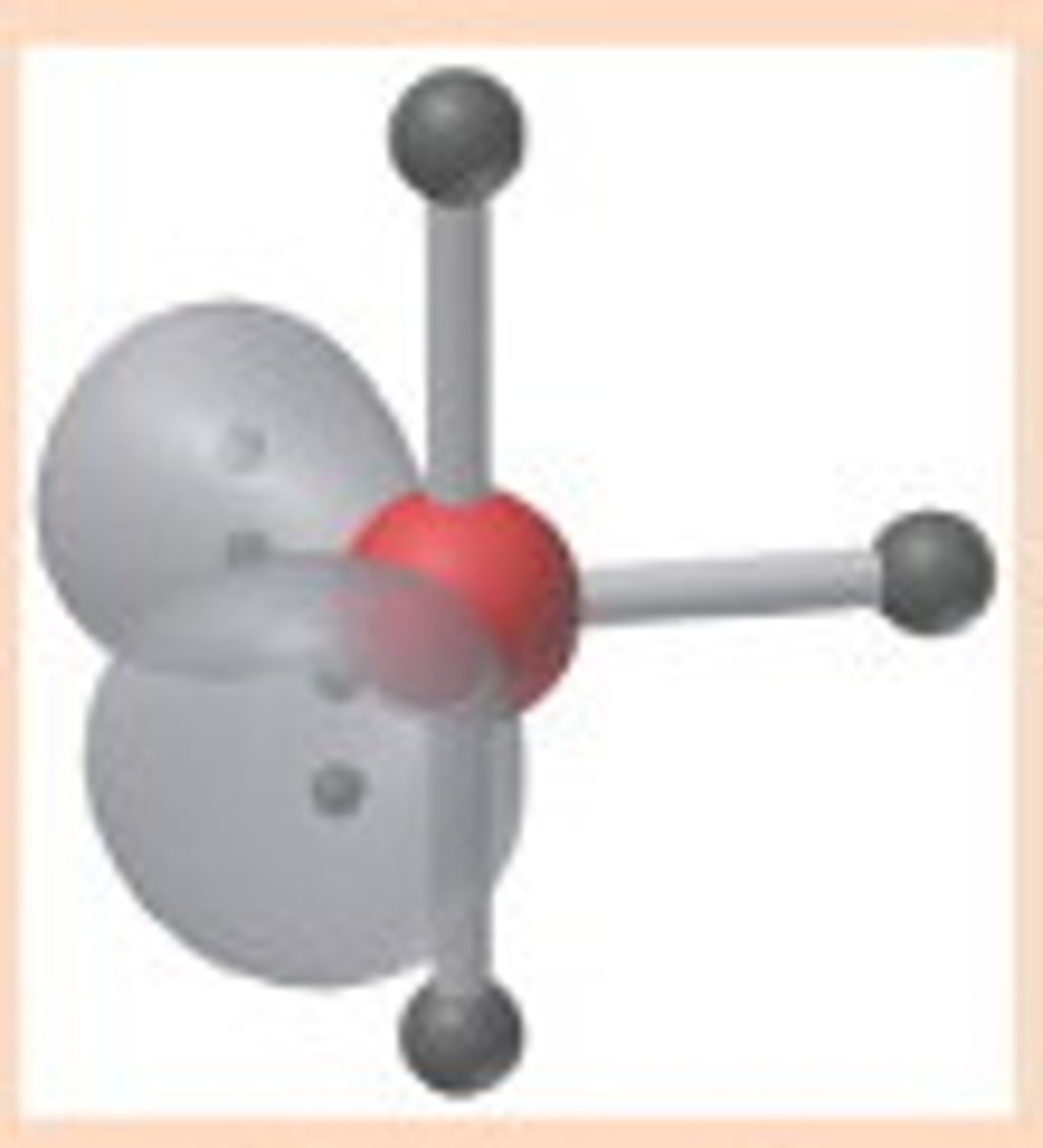
T-shaped angle
90 and 180
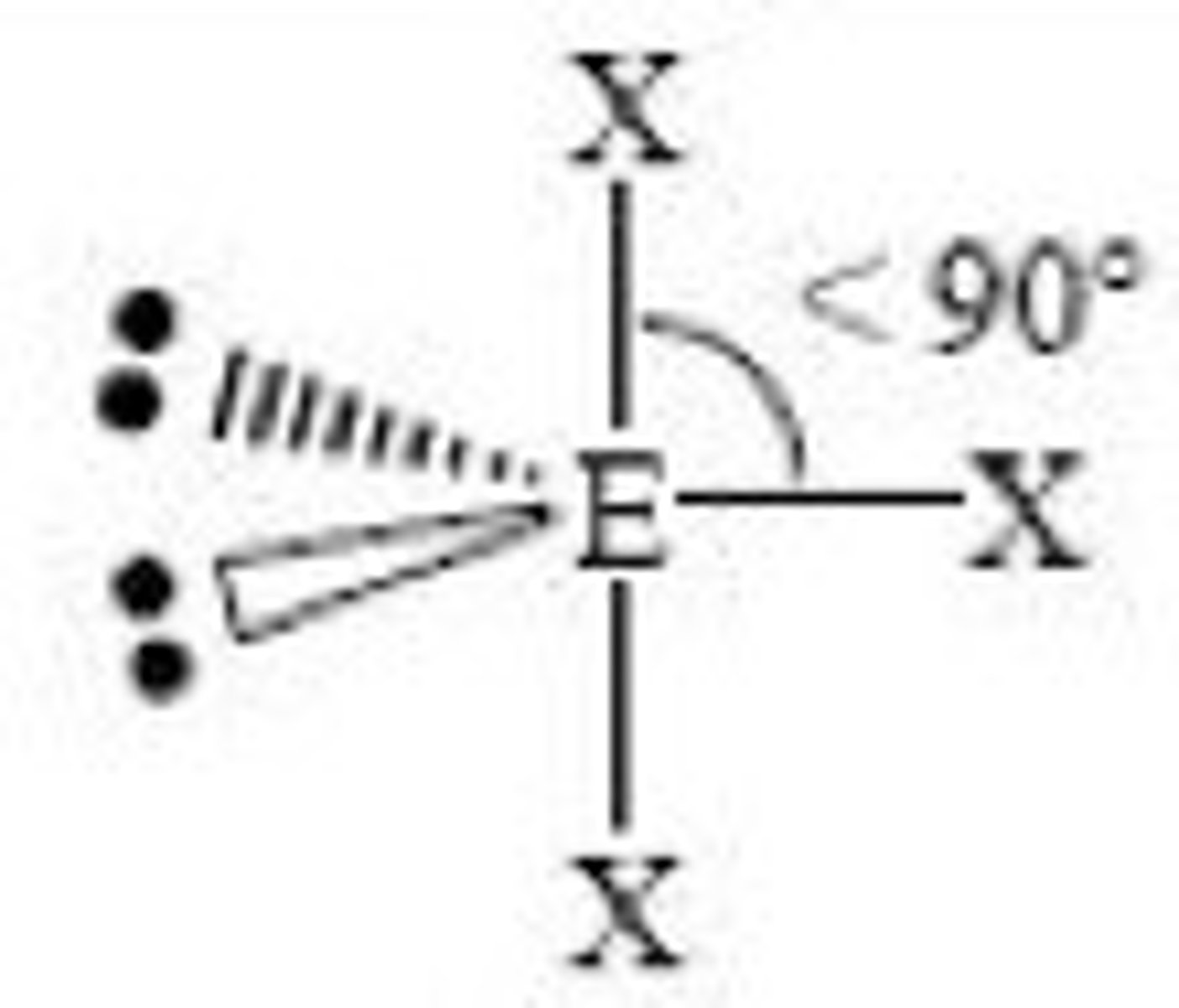
5 electron pairs, 3 lone pairs
linear
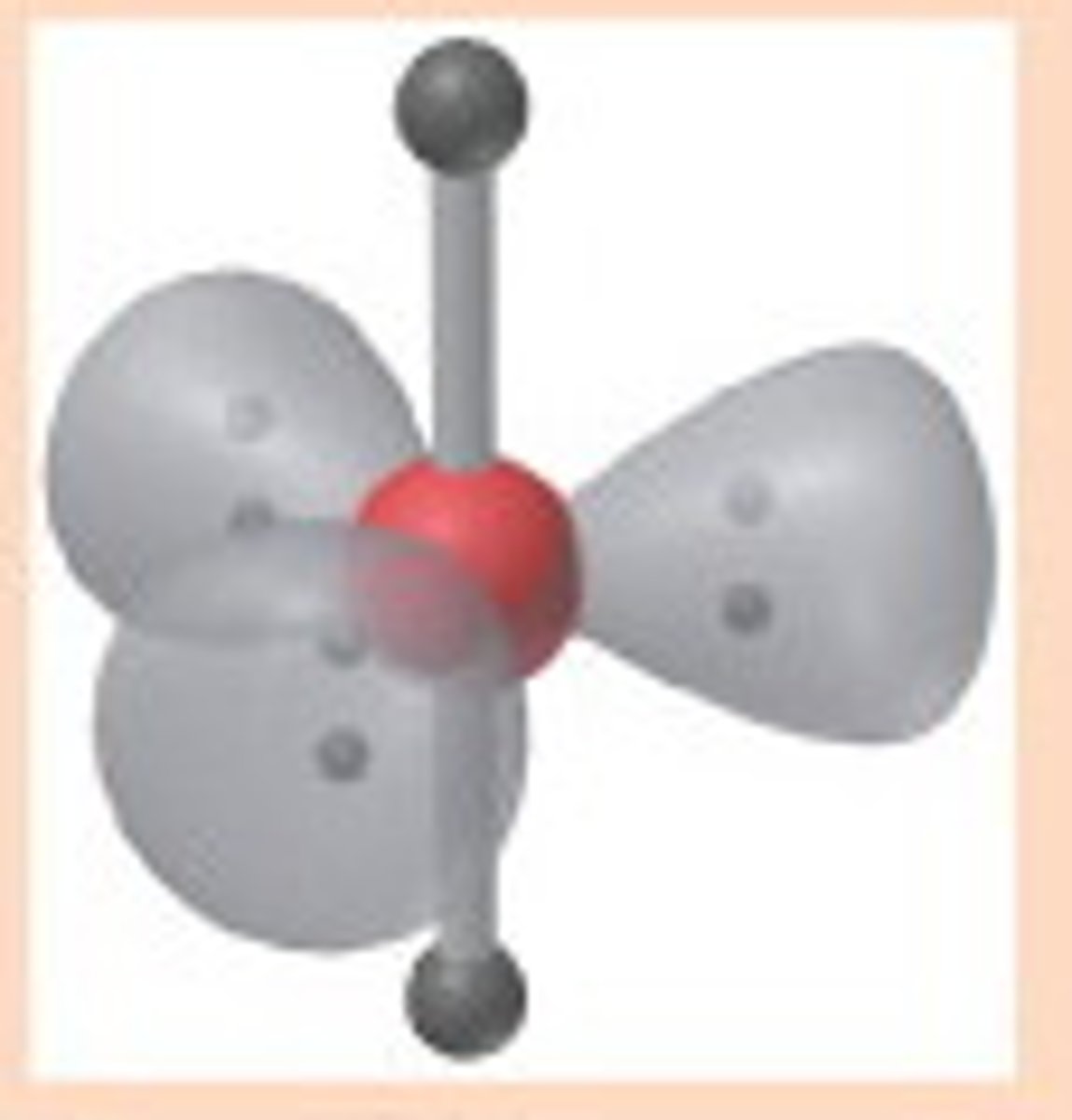
6 electron pairs, 0 lone pairs
octahedral
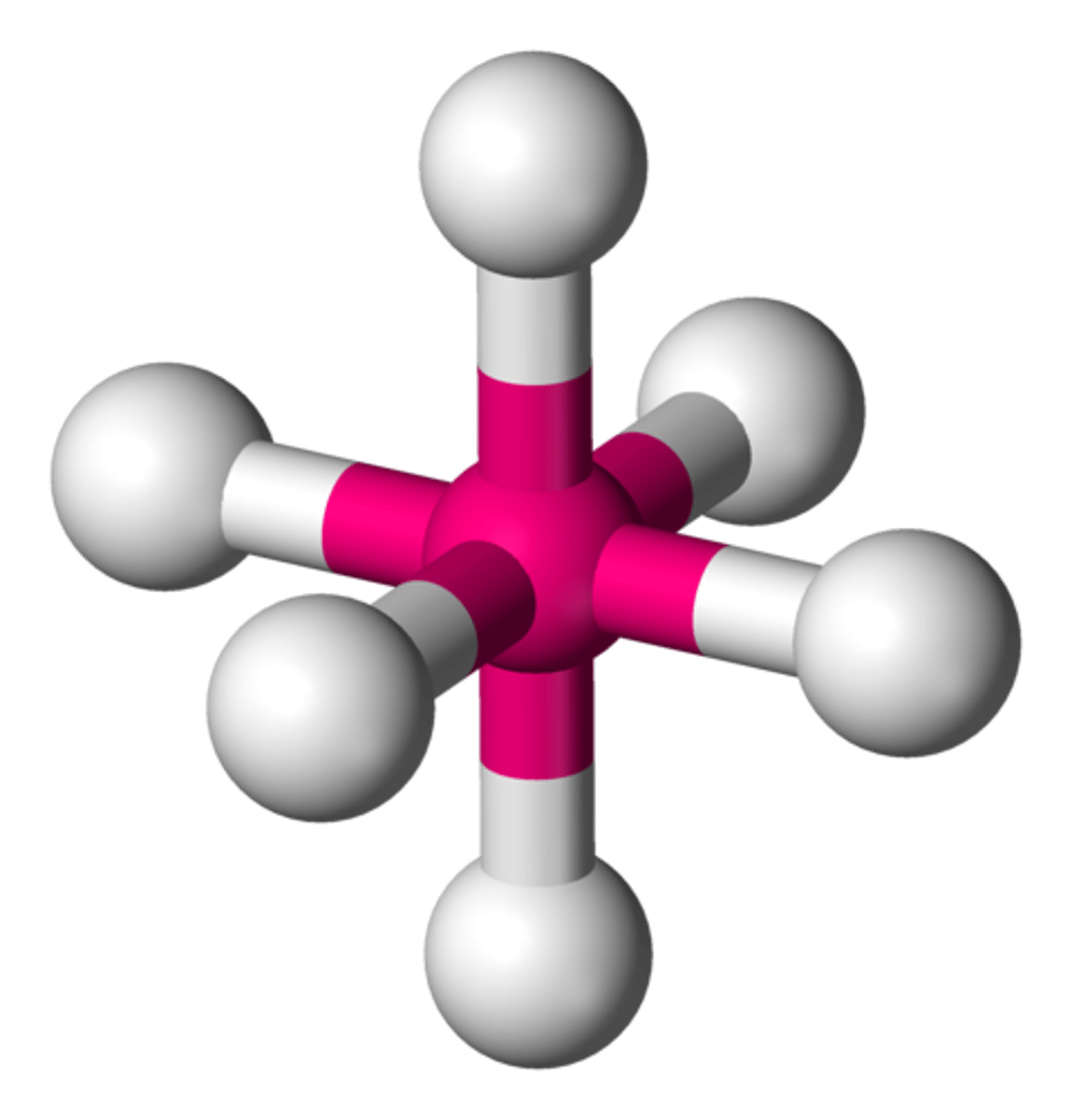
octahedral angle
90 degrees
octahedral forms what shape
a cube with the center at om in the middle and the outside atoms as the faces
trigonal pyramidal forms what shape
a cube with the H and electron pairs on opposite corners of the planes.
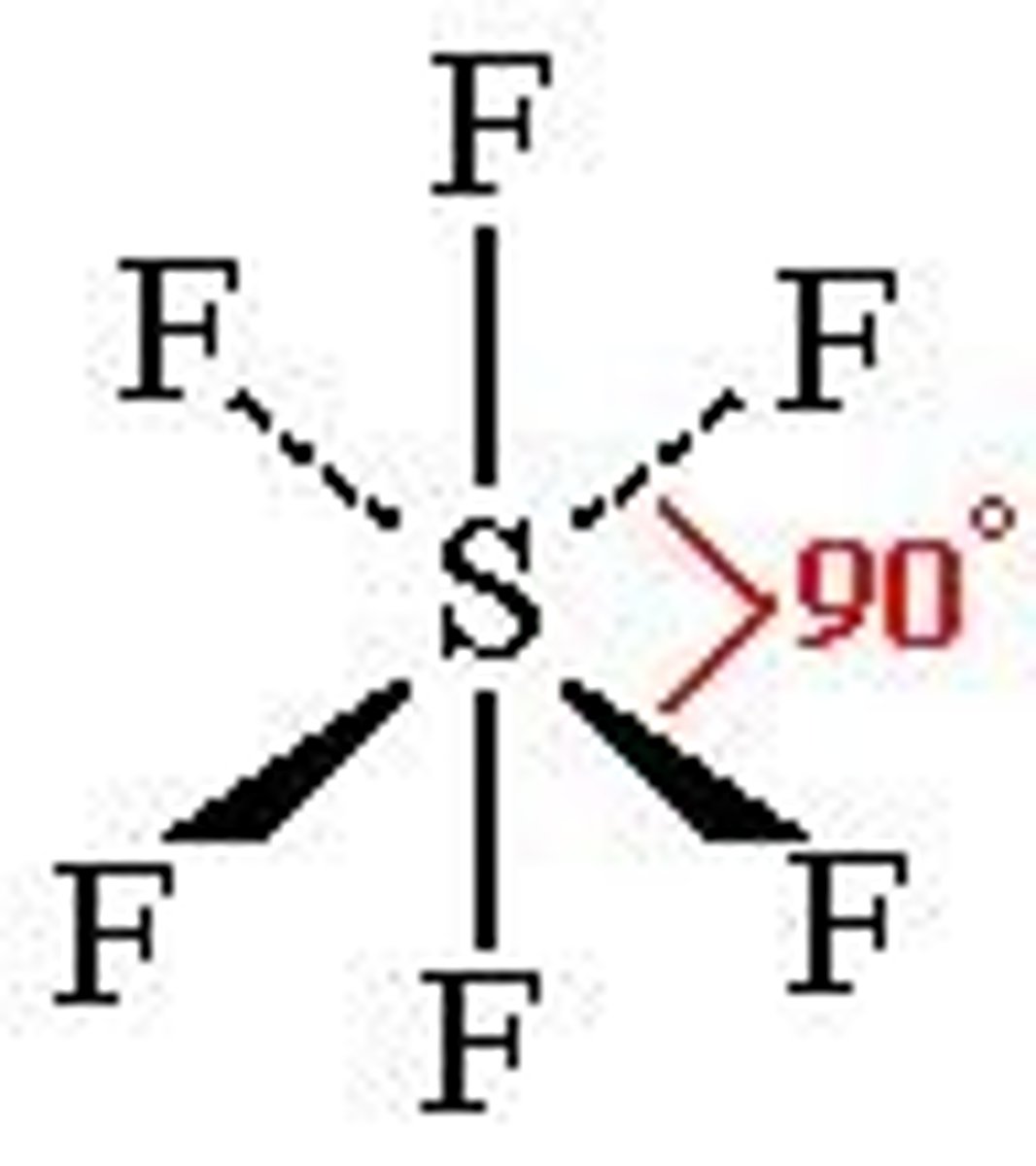
6 electron pairs, 1 lone pair
square pyramidal
square pyramidal angle
slightly less 90
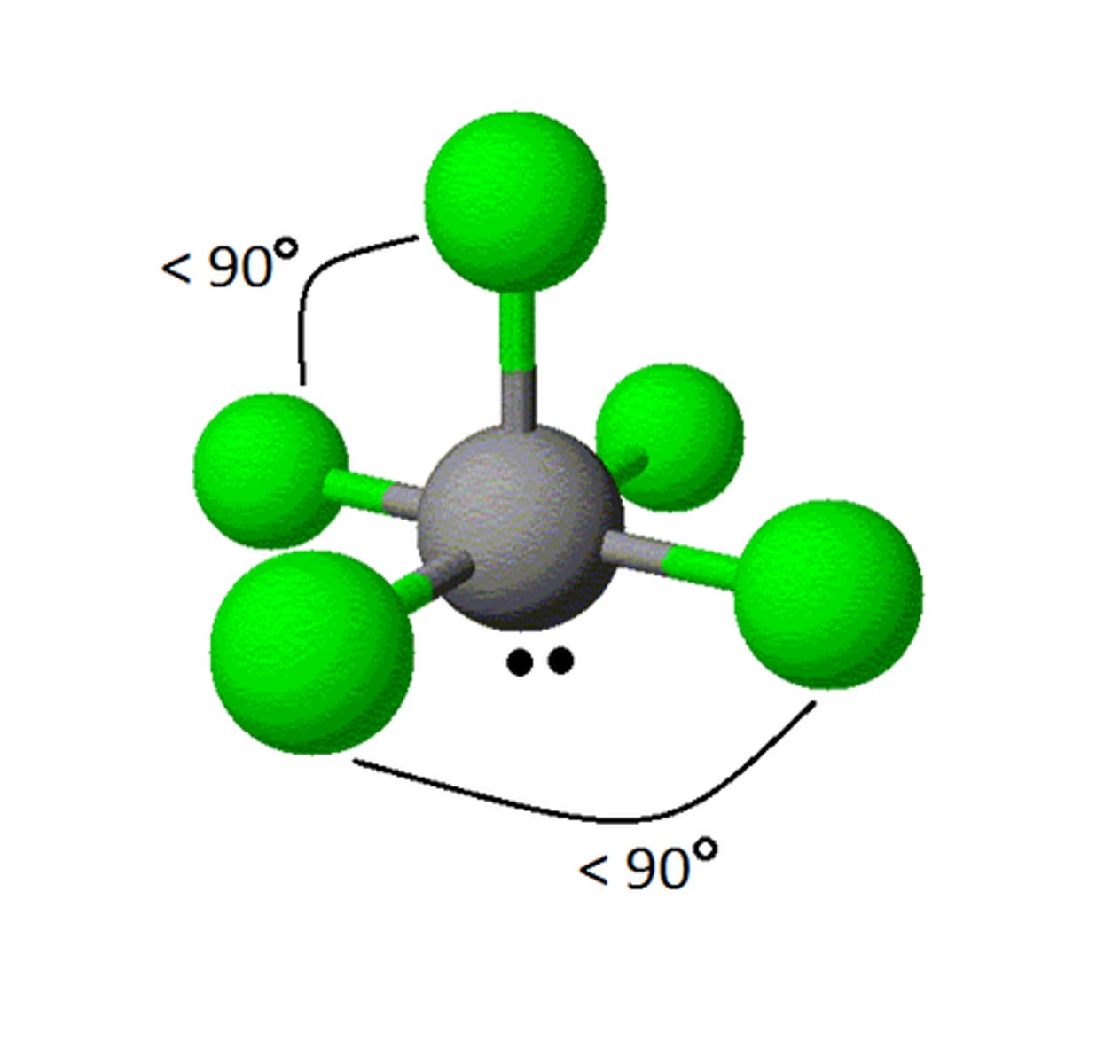
6 electron pairs, 2 lone pairs
square planar
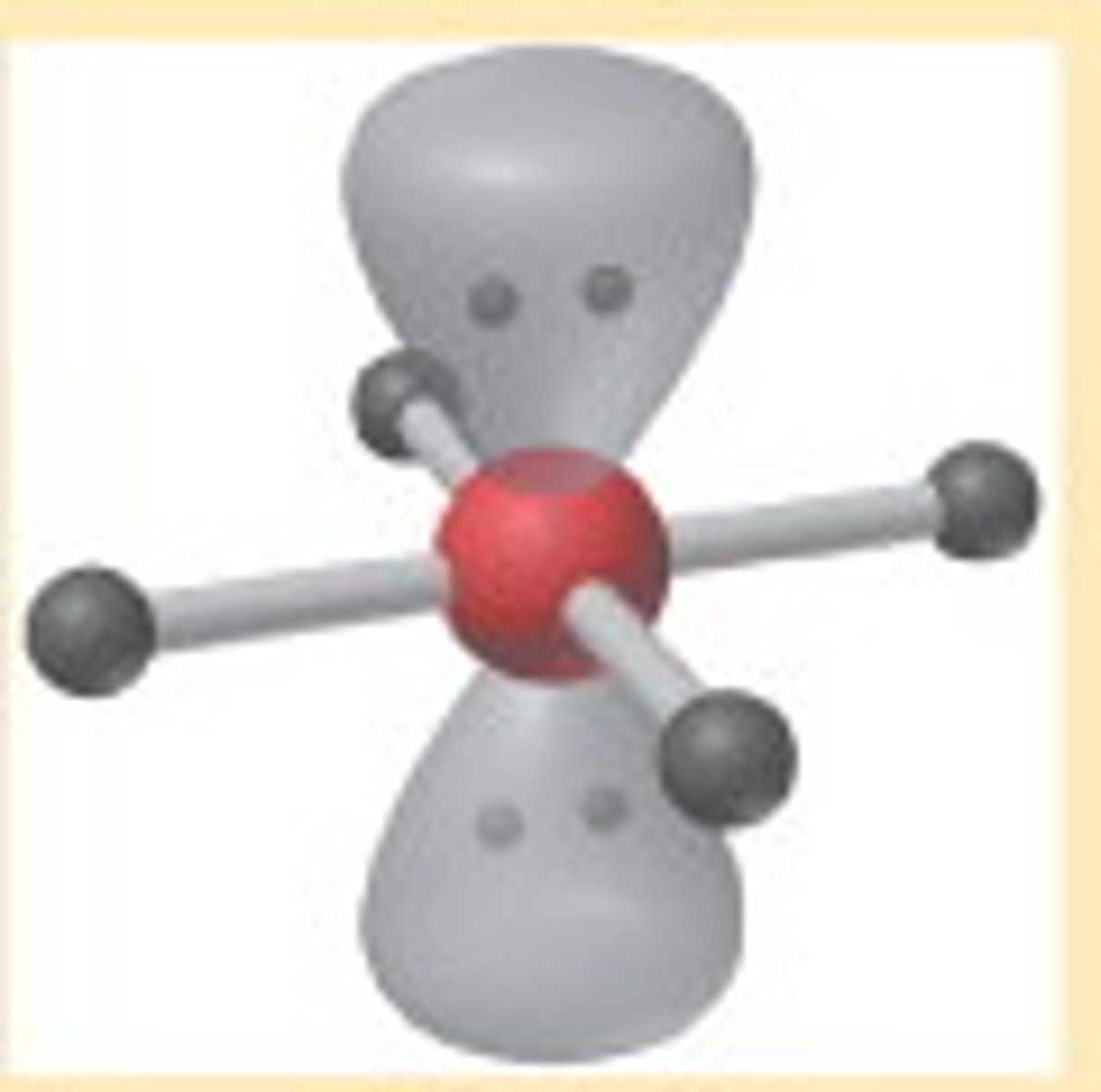
square planar angle
90 degrees
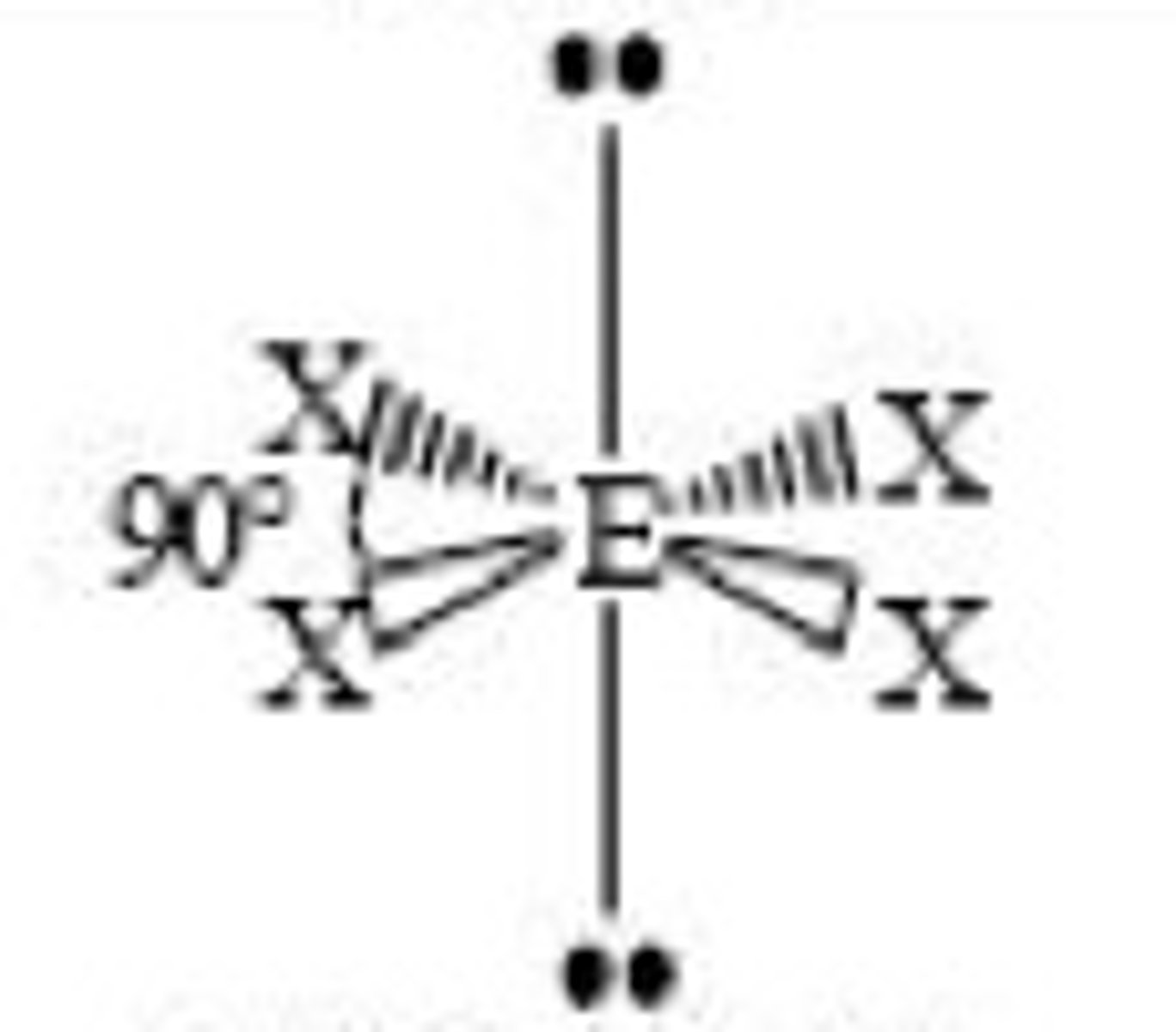
multiple bonds are the same as...
single bonds in VSEPR
molecular shape considers only...
atoms