Atomic Theory
1/7
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
8 Terms
name all the sublevels
s, p, d, f (remember spdf is vds)
how many e- does the s sublevel have and how many orbitals?
2 electrons, 1 orbital
how many e- does the p sublevel have and how many orbitals?
6 electrons, 3 orbitals
how many e- does the d sublevel have and how many orbitals?
10 electrons, 5 orbitals
how many e- does the f sublevel have and how many orbitals?
14 electrons, 7 orbitals
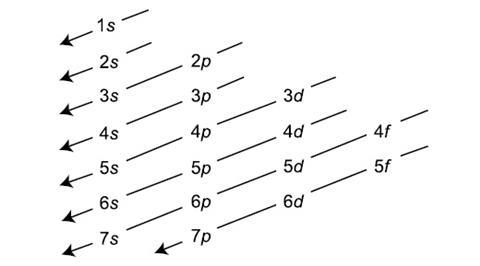
what is this?
aufbau’s principle pattern (helps determine the electron configuration)
4 atomic structures
electron config., orbital box diagram, energy level diagram, and bohr
2 Exceptions to the filling order
For d-block (transition metal) cations remove the outermost electrons in the s-orbital first, before removing any d-orbital electrons
Transition metals (d-block) that have 4, 8, or 9 electrons will STEAL electrons from the nearest s-orbital
Note: extra stability comes with a filled or half-filled sublevel