Week 8 - Parasitism
1/75
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
76 Terms
Symbiosis
The relationship where unlike microorganisms exist together
3 types of symbiotic relationships
1. Commensalism
2. Mutualism
3. Parasitism
Commensalism
Form of symbiotic relationships in which two species live together and one species benefits from the other without harming or benefiting the other

Example of Commensalism
Human body and Normal Flora
Mutualism
Is a symbiotic relationships in which two organisms mutually benefit from each others
Parasitism
Is a form of symbiotic relationships where one party or symbione benefit to the detriment of the other (like host)
- in most cases parasites deprives the host's essential nutrients and produce disease in the host
Parasite and host
Two important elements of parasitism
ectoparasites
endoparasites
Classification of parasites (Based on Habitats)
Ectoparasites
parasites that live outside the host’s body (e.g fleas, lice).

endoparasite
parasite living on the inside of its host (e.g helminths or worms)

Infestation
Invasion of the body by ectoparasites is called ___
Infection
Invasion of the body by endoparasites is called ___
Facultative parasite
parasites that can live independently of the host (i.e. free living). These parasites do not have to live inside a host to complete their life cycle (e.g. Stringylodies stercoralis)
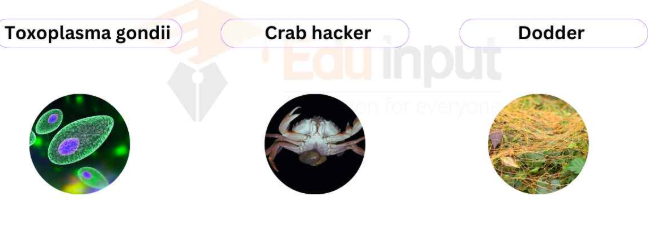
obligate parasites
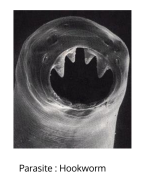
parasites that must live inside a host to complete the life cycle (e.g. Plasmodium Leishmania, hookworms). Majority of the parasites that infect humans are ___
Facultative Parasites
Obligate Parasites
Classification of Parasites (based on the abilities to live independently of the host)
Permanent parasites
parasites that remain in a host from early life to maturity (e.g Plasmodium)
Classification of Parasites (Mode of Living)
Permanent Parasites, Intermittent parasites, Incidental parasite, transitory parasites, erratic parasites
Intermittent parasites
parasites that simply visit the host during feeding time (e.g. non-pathogenic parasites)

Incidental parasite
Parasite occurs in an unusual host (e.g dog tapeworm in human)
Examples: Rat Tapeworms, dog tapeworms
Although they must affect rats/dogs, these parasites have been shown to affect human beings. (Cases are very low)
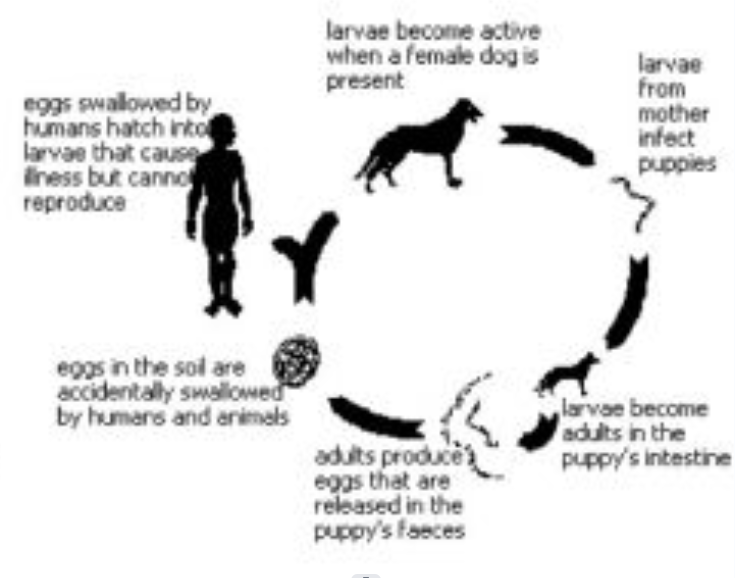
Transitory parasites
Parasites whose larva develops in a host while the adult is free-living (e.g. Echinococcus granulosus or dog tapeworm)
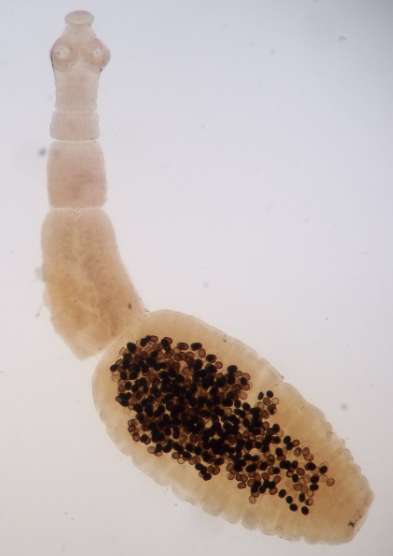
Erratic parasites
parasites that are seen in an unusual organ, different from that which it ordinarily parasitizes (e.g., Entamoeba histolytica in the lungs or kidneys).
Four types of Host
Definitive Host
Intermediate Host
Reservoir Host
Paratenic Host
definitive hosts
are hosts that harbor the adult stage of the parasite or where the sexual stage or sexual phase of the life cycle of the parasite occur
Intermediate Host
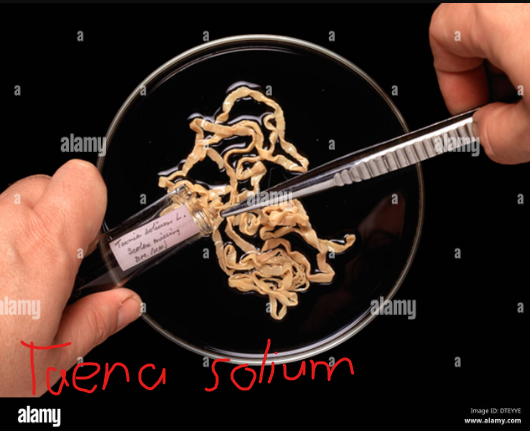
are those that harbor the larval stage of the parasite or where the asexual stage of the life cycle of the parasite occures(e.g the parastic worm, Taena solium (pork tapeworm).
For example, some tapeworms make use of cows, pigs, and fish as ___
Reservoir host
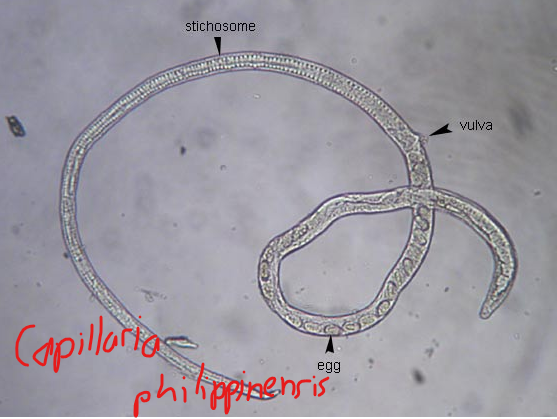
are vertebrate hosts that harbor the parasite and may act as additional source of infection in man.
Example migratory birds serve as the resevoir host for the parasite Capillaria philippinensis which people normally ger from contaminated fresh water.
Paratenic host
-are those that serve as means of transport for the parasite (e.g insect vectors) so that the infective stage of a certain parasite may reach its final host.
-are organism that harbors the sexually immature parasite’s development cycle to progress.

Sources of exposure to parasite
- Contaminated soil or water
- Food containing the parasite’s infective stage
- blood sucking Insect
- Domestic or wild animal harboring the parasite
- another person and his or her clothings, bedding, or the immediate environment he or she has contaminated
- one's self (auto-infection) (e.g Strongyloides stercoralis, Enterobius vermicularis, Taena solium, and Hymenolepis nana)
- soil contaminated or polluted with human feces
Ascaris lumbricoides
giant intestinal roundworm

Mode of transmission of parasites
-Ingestion of contaminated food and water(fecal-oral transmission) is the most common mode of transmission of most intestinal parasites
-Some parasites actively enter the body through penetration of the skin from the soil (e.g. hookworms and Strongyloides) or from contaminated water (e.g. blood fluke)
Other modes of transmission
- Bite of blood-sucking insect vectors
- Inhalation of eggs (pinworm or Enterobius vermicularis);
- Transplacental or congenital infection (Toxoplasma gondii and occasionally Plasmodium)
- Transmammary (mother's milk) infection (Strongyloides, Ancylostoma); and
- Through sexual intercourse (Trichomonas vaginalis)
Portal of Exit of Parasites
-The most common portal of exit of parasites is through the anus - eggs of roundworms are excreted together with human feces and contaminate soil and water
-Urine may serve as a portal of exist - eggs of roundworms are excreted together with human feces and contaminate soil and water
(e.g. Trichomonas vaginalis, Strongyloides stercoralis, and Schistosoma haematobium)
-Sputum from phlegm (lung fluke)
(e.g. Paragonimus westermani and intenstinal roundwor Ascaris lumbricoides (larval stage).
sputum
-mucous secretion from the lungs, bronchi, and trachea expelled through the mouth
-Used to find Paragonimus westermani, Strongyloides stercoralis (with hypertension), E. histolytica, Ascaris lumbricoides larva, and the larvae of hookworms
Pathogenesis
Dynamics of any disease process.
-Some parasites may cause inapparent infection, causing no symptoms, and producing no detectable harm.

Traumatic Damage
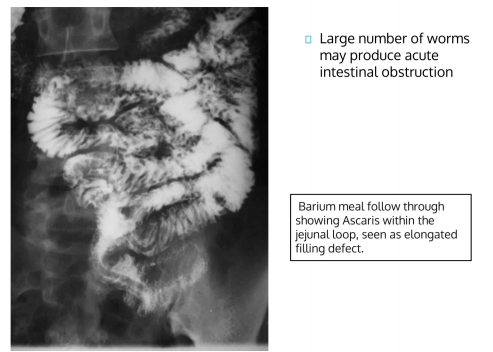
In this mechanism of damage, the manifestations may be due to the direct physical damage caused by the parasite in the organ it parasitizes or at the point of entry of the parasite.
Entry of the infective larvae of hookworms or blood flukes into the skin may produce relatively slight physical damage.
Lytic necrosis
Enzymes and other substances produced by many parasites that are necessary for them to digest food available in the immediate environment may cause harm to the host tissues.
Ex. Protozoan Entamoeba histolytica which releases enzymes that lyse tissues for their nutritional needs - these enzymes also enable the parasite to penetrate the tissues of the colon, producing ulcerations in the colon, and extra-intestinal viscera.
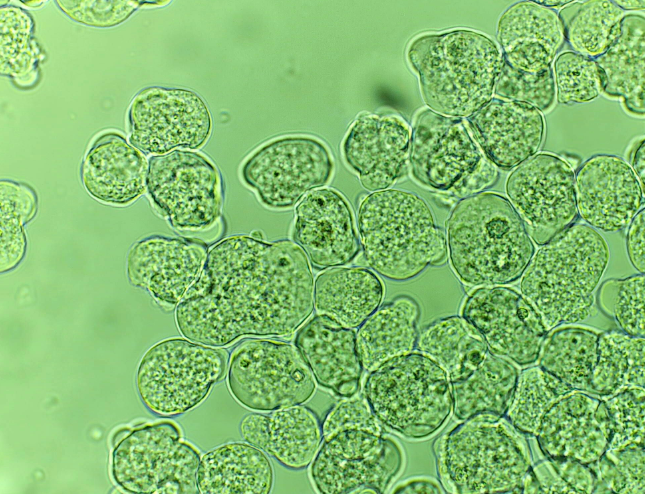
protozoan entamoeba histolytica
transmitted in feces-contaminated food and water
causes amebiasis, also called amoebic dysentery
-Causes Lytic Necrosis
Stimulation of host tissue reaction
Systemic increase in certain types of cells - circulating in the blood may increase, Eosinophils: true for most infections caused by helminths
In some cases, stimulation of RED BLOOD CELLS production may occure - (e.g. hookworm infection or in malaria)
Certain parasitc infection may lead to stimulation of neoplastic (cancer) growth in the organ infected.
-e.g blood fluke Schistosoma japonicum may lead to cancer of the liver; live fluke Clonorchis sinensis lead to cancer of the biliary ducts
Neoplasm
new growth (tumor)
Scientific name of liver fluke
Fasciola hepatica
Toxic and Allergic Phenomena (Immunopathology)
Proteins or other metabolites produced by the parasites may lead to hypersensitivity or allergic reactions due to stimulation of antibody production.
Ex: infection with pinworm Enterobius vermicularis - allergic reaction occurs in the anus as a response to the female worm and its eggs leading to its most prominent manifestation of pruritus ani.
Opening of Pathways for entry of other pathogens into the tissues
The presence of the parasites and the damage they produce to the tissues may favor the entry and proliferation of other organisms, especially bacteria
For instance, infections with pinworm leads to intense itchiness in the anus - scratching - leading superficial erosions - point of entry for bacteria - causing secondary bacterial infection.
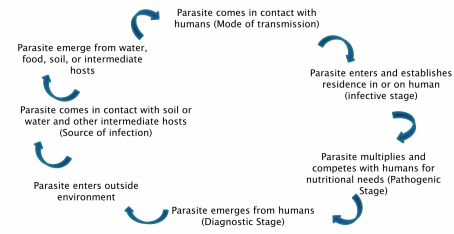
Life Cycle of Parasites
- Simple to the most Complex
Its components are - Source of Infection, mode of transmission, the infective stage (morphologic form that is responsible for pathology produced leading to clinical manifestations) and the diagnostic stage (morphologic form that can be detected through laboratory methods).
Classification of Parasites
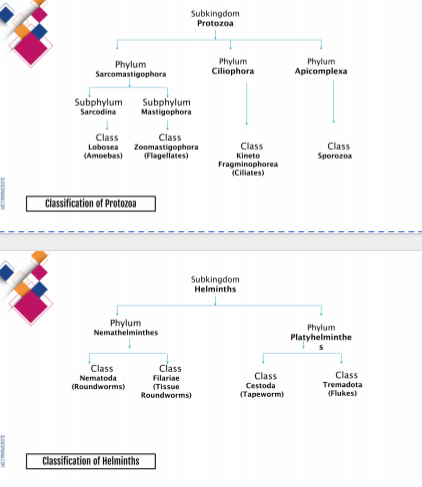
Parasites may be classified into 2 major groups: the single-celled Protozoa (sub-kingdom Protozoa) and the multicellular metazoa (sub-kingdom Metazoa) called Helminths.
The pararastic protozoa are further classfied into 4 groups based on their means of motility and mode of reproduction: sporozoa (phylum Apicomplexa) and ciliates (phylum Ciliophora).
The parasitic helminths or worms are subdivided into two phyla: Nemathelminthes (roundworms) and Platyhelminths (flatworms). THe flatworms are xomposed of 2 classes: Trematoda (flukes) and Cestoda (tapeworms)
Nemahelminthes
Phylum of roundworms
Platyhelminthes
Phylum of flatworms
Pseudopod
A "false foot" or temporary bulge of cytoplasm used for feeding and movement in some protozoans.
Laboratory diagnosis of parasitic infection
Specimen Collection, Handling and Transport
Microscopic Examination
-
Specimen Collection, Handling and Transport
A. Knowing the proper laboratory diagnostic procedure to request:
Includes knowledge of the proper specimen to collect
Specimen varies depending on the portals of entry and exit of the parasite
For instance, mouth - most common portal of entry
Anus - most common portal of exit: proper specimen is the stool
Multiple specimens may be needed for adequate detection
Ex. Three specimen collections, one specimen collected every other day
For suspected amoebiasis, up to 6 specimens collected within a period of 14 days is recommended.
B. Timing of Specimen Collection
To demonstrate the motility characteristics of a protozoan parasites, fresh specimen must be used.
The diagnostic stage for most protozoans is the trophozoite, which is usually found in liquid stool.
Liquid stools must be examined within 30 minutes after collection
Formed stools which usually contain the cyst forms may be held for a maximum of 24 hours after collection.
Preservatives or fixatives may be added if the specimen cannot be examined right away.
Ex. formalin, polyvinyl alcohol, sodium acetate formalin, and modified polyvinyl alcohol.
c. Specimen Collection
stool specimens must be collected in a clean, water-tight container and should be covered lightly
Approximately 2-5 g of stool is recommended
No contamination of urine
Stool should not be collected from water or from the toilet bowl. (eg. Ova of schistosomes and trophozoites of amoeba)
d. Proper Labelling
Proper labelling of the container must be observed - accompanied by fully accomplished request form.
Information other than patient general data may be included such as history of travel and clinical findings
Once specimen container is properly sealed, it must be placed in a ziplock plastic bag for transport to the laboratory
Universal precautions must be observed when handling all specimens - gloves should be worn at all times
Microscopic Examination
All fresh specimens submitted for examination must undergo microscopic examination - which is divided into three stages - direct wet preparations, concentration technique and use of permanent stains.
Ideally, the microsocope to be used must be equipped with an ocular micrometer since size (measured in microns or um) is an important diagnostic feature.
Direct Wet Preparation or direct wet mount
Purpose: To detect the presence of motile protozoan trophozoites; other stages detected include cysts, oocysts, ova and larvae of worms.
Principle: A small portion of unfixed stool is mixed with saline or iodine then studied under the microscope.
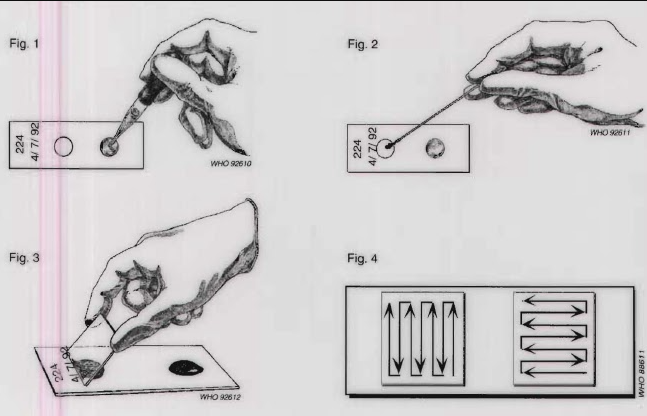
Procedure
Place small amont of unfixed stool on a glass slide.
Add a drop of 0.85% saline
Mix using a wooden applicator stick
Place a cover slip on the slide
Examine slide using both low-power and high-power objectives of the microscope
Concentration methods
Purpose:
To aggregate parasites prent into a small volume of the sample that enables the detection of small numbers of parasites that might not be detected in direct wet preparation
To remove debris and other contaminants that might interfere with the microscopic examination
It can be used on both fresh and preserved specimens. It is not done if the purpose is to detect the motile trophozoites since the trophozoites do not survive the procedure.
It can be used to detect cysts, oocysts, ova, and larvae of nematodes. Two types of concentration techniques are available - FLOTATION and SEDIMENTATION
Sedimentation (Formaline-Ethyl Acetate Sedimentation Procedure (Widely Used))
Principle:
This is based on specific gravity - parasite are heavier than the solution used and thus settle in the sediment of the tube while the fecal debris which are lighter will rise to the upper layers of the test tube.
Procedure:
Ethyl acetate is added to a saline-washed formalin-fixed sample in a test tube and then centrifuged
Advantage:
It provides good recovery of most parasites and it is relatively easy to perform
Disadvantage:
The preparation contains more fecal debris than a floatation technique
Zinc Sulfate Flotation Technique
Principle:
This is based on the differences in specific gravity and the sample debris (in this case heavier thus sinks to the bottom while the parasite is lighter and thus floats upwards the top of the tube)
the zinc sulfate used has a specific gravity of 1.18-1.20 and is used as the concentrating solution
Advantage:
It is able to remove more fecal debris, hence will yield a cleaner preparation
Disadvantage:
Some helminth eggs are denser and may not float to the upper layer of the test tube.
Permanent Stains
This serves as the final step in the microscopic examination for the detection of parasites
A small amount of the fixed sample is placed on a slide glass and allowed to dry after which it is stained
A cover slip is then placed after which a sealant is applied, thus allowing the sample to remain intact for a longer period. It is designed to confirm the presence of cysts and/or trophozoites of protozoans
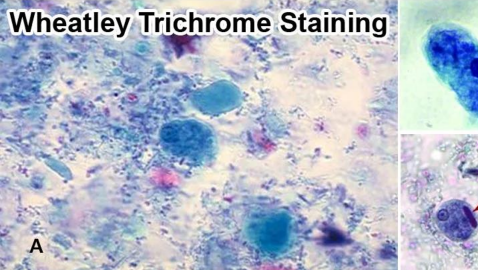
Stains may be used include Wheatly trichome (most widely used), iron hematoxyline (to demonstrate morphology of intestinal protozoa), and other specialized stains (e.g. modified acid fast stain to detect oocysts of Cryptosporidum).
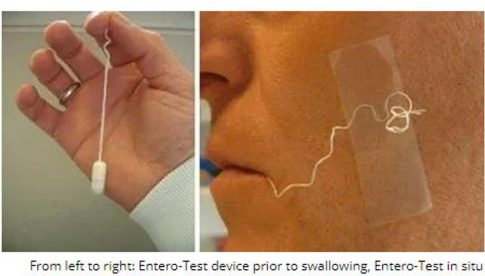
Duodenal Material
This may be collected using a nasogastric tube (NGT) or through the enteric capsiule test (Entero-test).
The collected duodenal fluid must be examined immediately (rapid deterioration of trophozoites)
Volume of > 2 mL is recommended
Sample undergoes centrifugation prior to microscopic examination of the sediment.
In the Entero-test, the patient is made to swallow a gelatin capsule that contains a coil of yarn that is weighted
The yarn is released and is carried to the duodenum as the capsule dissolves in the stomach.
The free end of the yarn is attached to the patient’s neck or cheel and after an incubation of 4 hours is pulled back out of the patient.
The bile-stained material attached to the string is then examined microscopically with wet preparation followed by the application of permanent stains
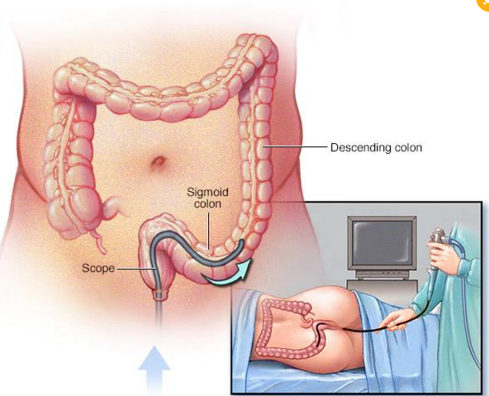
Sigmoidsocopy Material
Is used to collect and examine material from the colon.
Helpful in diagnosis of infection with Entamoeba histolytica. - Biopsy of colon material may be done
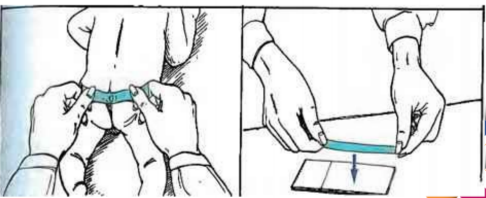
Cellophane Tape or Scotch Tape Preparation
This proecure is done to detect eggs of the pinworm Enterobius vermicularis. The female parasite migrates tp the anus at night where it lays its eggs
The procedure must therefore be done first thing in the morning, before the patient defecates or washes.
It may also be used to detect the eggs of the tapeworm Taenia spp.
Blood
Examination of blood can detect the presence of blood-borne parasites such as Leishmani, Trypanosoma, Plasmodium, and the fillarial worms
Univeral precautions and asepsis must be observed during the collection and handling of blood specimen.
Blood from the fingertip of earlobe may be used (without anticoagulant) or from standard venipuncture (with anticoagulant).
In cases of suspected malaria infection, thick and thin blood smears must be prepared and examined within 1 hour of collection
Thick smear - screening purposes and used when parasites in the red blood cells, important in species identification
Thin smear - demonstrate the malarial parasites in the red blood cells, important in species identification
> The prepared smears then be stained with Wright’s stain or Giemsa stain
Leishmani, Trypanosoma, Plasmodium, and the fillarial worms
Blood-borne parasites
Thick smear
screening purpose of rbc and used when parasites are few in number
Thin smear
Demonstrate the malarial parasites in the red blood cells, important in species identification
Cerebrospinal Fluid (CSF)
It may be used to diagnose certain amoebic infections. It may also be used in patients with African sleeping sickness.
Similar to blood, it must be immediately examined if detection of parasite motility is desired
Wet preparations can be done to detect characteristics morphologic forms of Naegleria, Acanthamoeba, and Trypanosoma as well as Toxoplasma gondii, Taenia solium (cysticercosis), and Echinicoccus.
Tissue and biopsy specimens
This may be utilized to detect the presence of Leishmania, Toxoplasma gondii, Trypanosoma, Taenia solium, and Trichinella spiralis in tissues
In patients with suspected amebic liver abscess, the abscess material taken from the liver is the specimen of choice.
Genitourinary Secretions
The specimen of choice for detecting the blood fluke Schistosoma haematobium is urine. It may also be used to detect Trichomonas vaginalis, which may be isolated from genital secretions
Urine samples are centrifuged and the sediments examined for the presence of the parasites
Genital secretions may be collected using a sterile cotton swab.
Saline wet preparation is then performed to demonstrate the trophozite of the parasites.
Eye specimens
Acanthamoeba keratitis, Toxoplasma gondii, and Loa loa
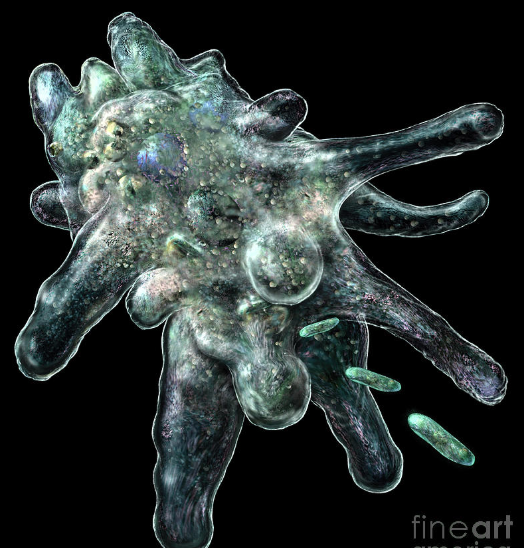
Amoeba
Unicellular; cyst and trophozoite forms
binary fusion
Pseudopods
Facultative anaerobe
Assimilation by pinocytosis or phagocytosis
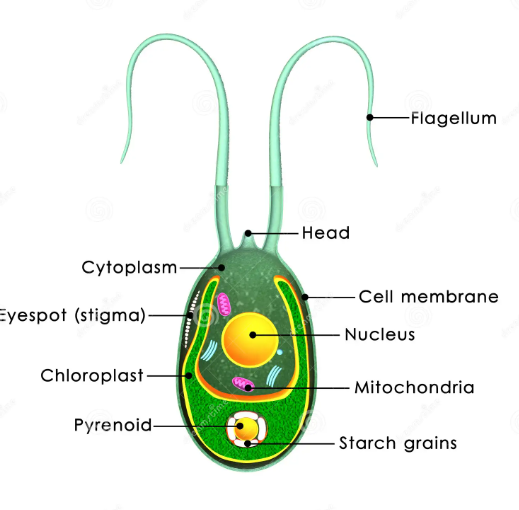
Flagellates
Unicellular; cyst and trophozoite forms
binary fusion
Flagella
Facultative anaerobe
Simple Diffusion or ingestion via cytostome, pinocytosis, or phagocytosis
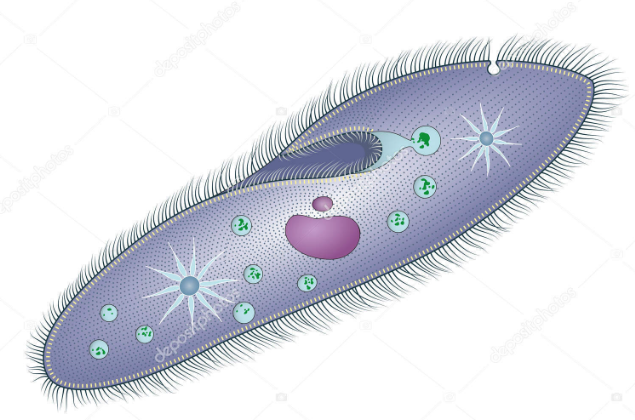
Ciliates
Uniceullar; cyst and trophozioite forms
Binary Fission or conjugation
Cilia
Facultative Anaerobe
Ingestion via cytostome, food vacuole
Sporozoa
Unicellular; cyst and trophozoites forms
Schizogony and sporogony
Facultative Anaerobe
Simple Diffusion
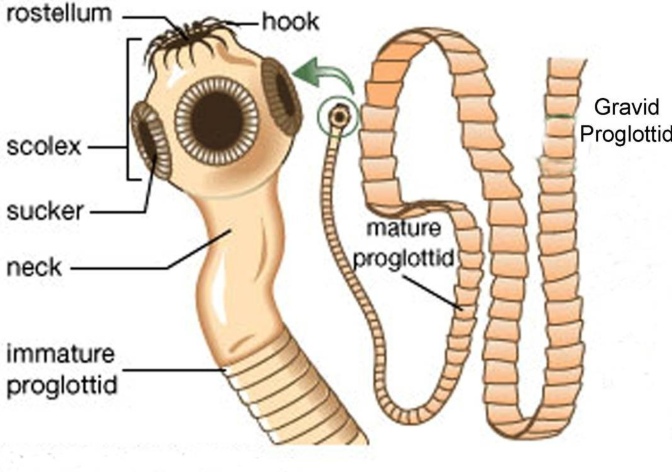
Cestodes
Multicellular, head with segmented body (proglottids); lack of digestive tracks; head equipped with hooks and or suckers for attachment
Hermaphroditic
No single organelle, usually attachment to mucosa; possible muscular motility (proglottis)
Adults usually anaerobic
Absorption of nutrients from intestines
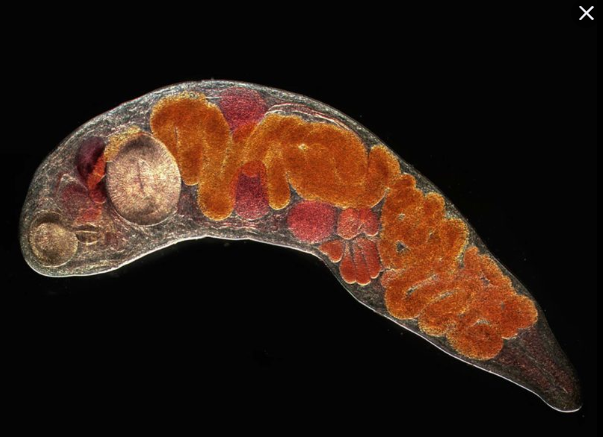
Trematodes
Multicellular, leaf shaped with oral and ventral suckers, blind alimentary tract
Hermaphroditic; Schitosoma spp. has separate sexes
No single organelle; muscle-directed motility
Adults usually anaerobic
Ingestion or absorption of body fluids or tissue or digestive contents
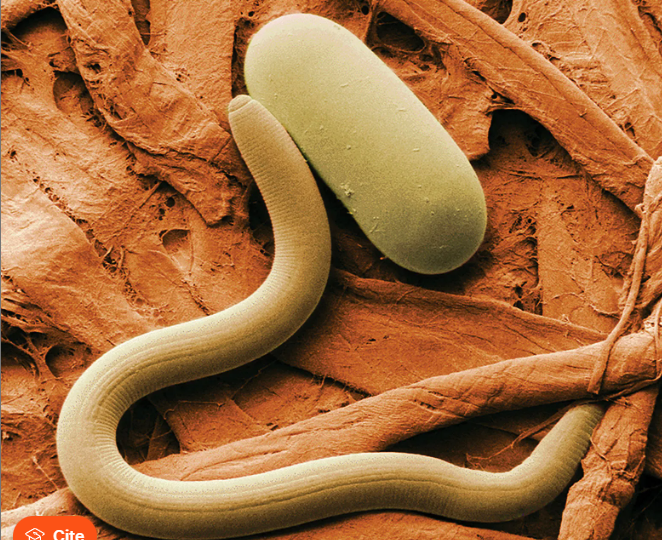
Nematodes
Multicellular, round, smooth, spindle-shaped, tubular digestive tract, possibly of teeth or plates for attachment
Separate sexes
No single organelle; active muscular motility
Adults usually anaerobic larvae possibly aerobic
Ingestion or absorption of body fluids, tissue, or digestive contents
Direct iodine wet preparation
variations of direct wet preparation include addition of a drop of iodine (Lugol’s or D’ Antoni’s) to enhance the detail of protozoan cysts
Mouth scrapings and nasal discharge
E. gingivalis, Trichomonas tenax, Naegleria fowleri
Skin snips
Skin fluid without bleeding obtained by making a small cut into the skin with a razor blade; to detect motile microfillariae
Xenodiagnosis
a special method for diagnosis of Chagas disease where an unifected reduviid bug (the vector) is allowed to take a blood meal from an infected patient and the feces of the bug is then examined for the presence of Trpanosoma cruzi