Factors that effect enzyme activity
1/21
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
22 Terms
What can the rate of an enzyme catalysed reaction be affected by?
Enzyme concentration
Substrate concentration
Temperature
pH
Presence of inhibitors
Measuring enzyme controlled reactions- initial rate of reaction
Rate= formation of a product in a given time
Reactions are always fastest at the start
When the enzyme and substrate are first mixed there is greater chance of collisions with the active site
As reaction proceeds, substrate molecules have been used up and less are available to the active sites
As a result, the frequency os collisions decreases so the rate of reaction gives the maximum reaction rate for an enzyme
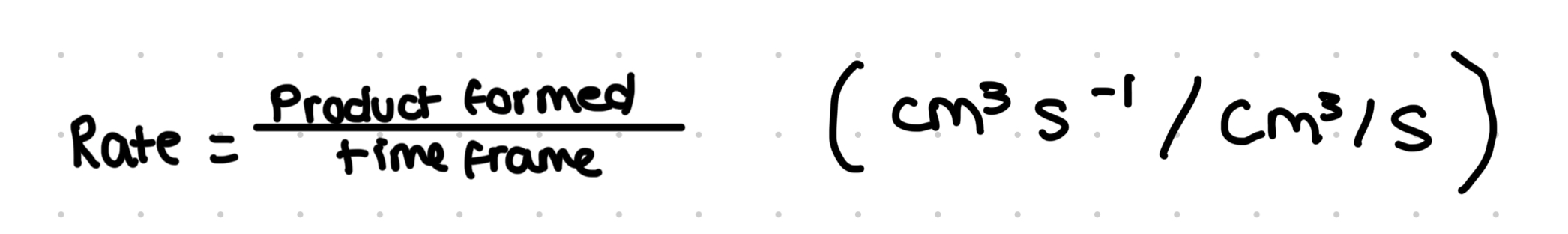
Effect of enzyme concentration
As enzyme concentration increases, so does initial rate of reaction
So long as there is sufficiently excessive substrate then the rate of a reaction will increase proportionally to the enzyme concentration
This is because there are more enzymes for the substrate to collide with, forming more ESC’s and catalysing more reactions
Effect on enzyme concentration graph
Rate plateaus is sub conc is fixed- sub conc is the limiting factor
Linear relationship- directly proportional- as one increases, so does the other- enzyme conc is limiting factor
As enz conc increases, more available active sites to catalyse reaction, so rate of reaction increases
Effect of enzyme concentration on living cells
In living cells, the availability of enzymes depends on the the rate of synthesis/ degradation
Genes for synthesising enzymes can be turned on/off depending on the cells needs
Old enzymes are degraded by the cell into amino acid and used to synthesise new enzymes (enzymes don’t age- refers to recycling molecules)
This also removes proteins which are abnormally shaped and regulates metabolism of the cell
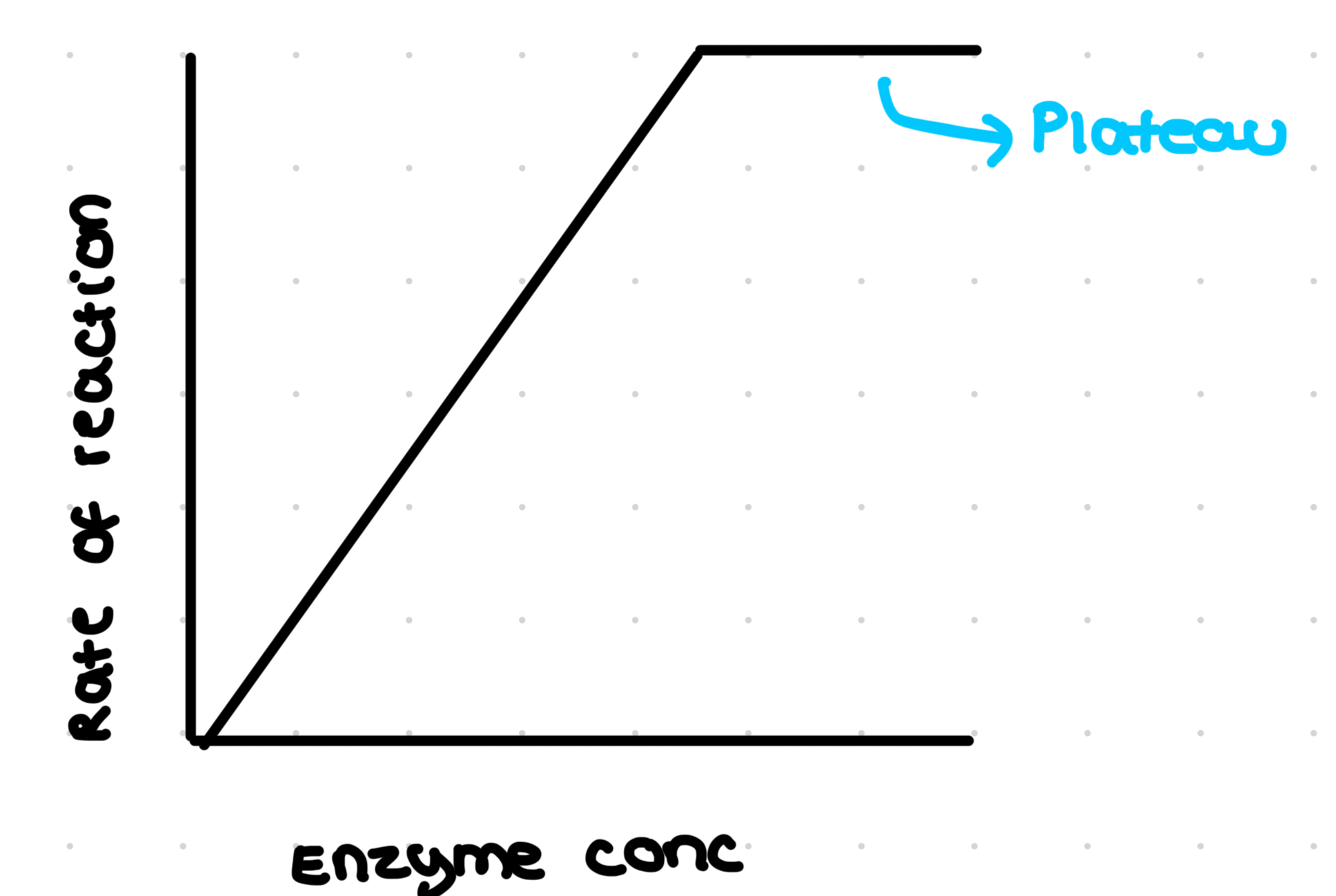
Effect of substrate concentration
Limiting factor- any factor that is determining how quickly a reaction is taking place
Initial rate os measured over 30p seconds for different substrate concentrations
The rate rises to a maximum (A) and then slows and levels off: plateaus (B) Because all active sites are occupied so rate does not increase
A- rate increases as substrate concentration increase so substrate is limiting factor
B- enzyme conc becomes limiting as all active sites are filled (known as V max), rate would increase again if there were more available enzymes
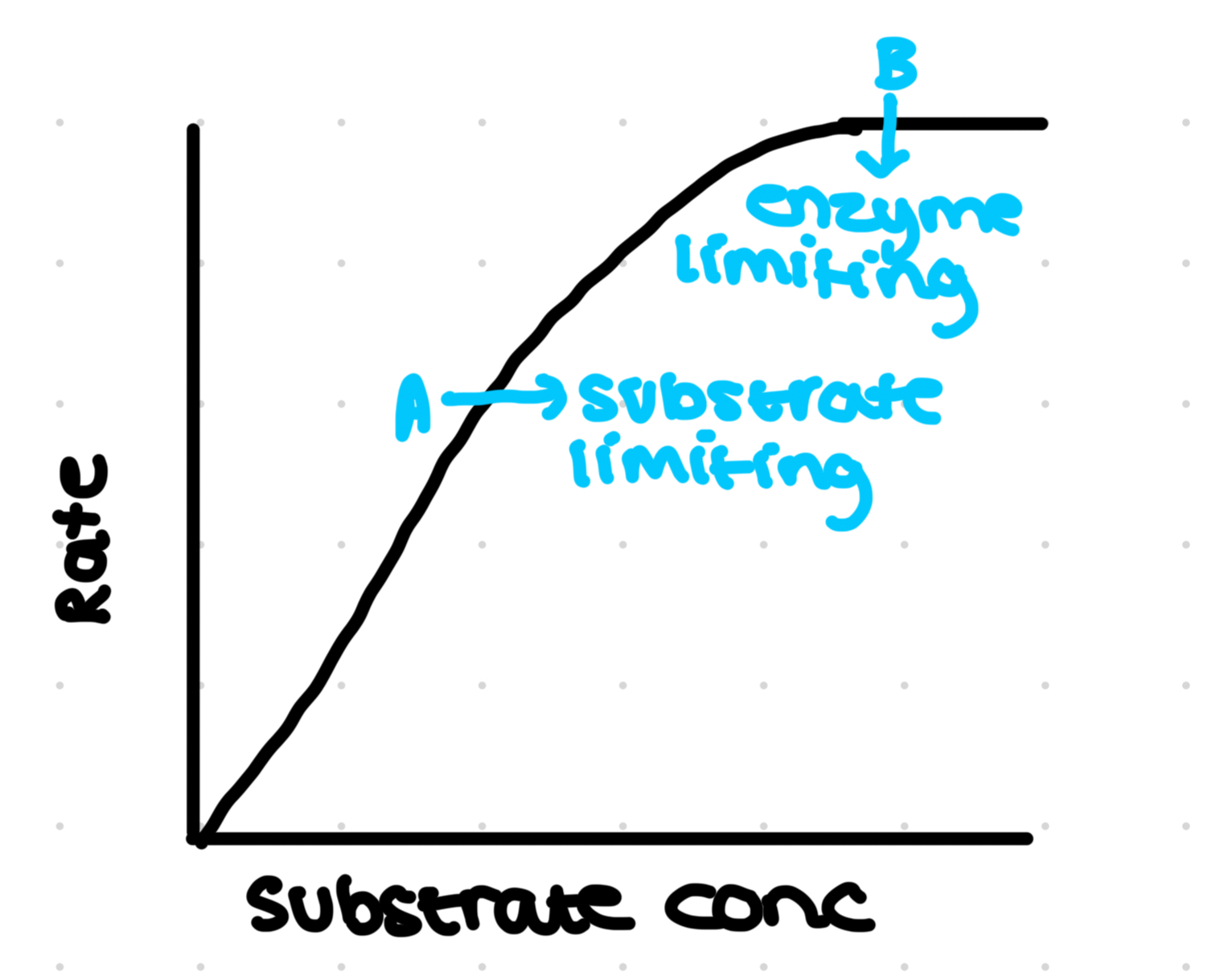
Effect of temperature
At low temperatures, increase temperature increases rate of facet ion due to increase kinetic energy of molecules:
Low energy- low collisions- not enough Ek
Heating up- more successful collisions with the required activation energy
However, at high temps a decrease in activity observed due to thermal desaturation of enzymes
Temperature: optimums
Our mammalian enzymes work at 37 degrees c (not all enzymes)
Range is specific to organisms and where they live
For some microbial enzymes, particularly those in thermophilic bacteria, optimal temp is as high as 65 degrees c
Temp is often given as a range as pinpointing the exact optimum is challenging
Temperature: link to proteins
Enzymes are globular proteins whose structure and active sites is determined by bonding between R groups
High temps = H-bonds vibrate more in the molecule itself between R groups, causing them to break, the active sites shape changes so the substrate no longer fits, and a reaction doesn’t occur (tertiary structure is disrupted)
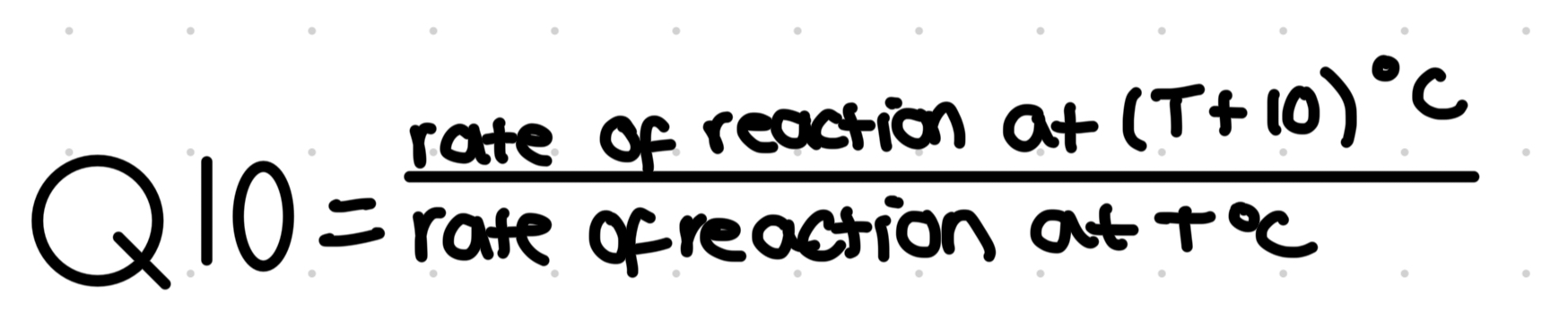
Q10- the temperature coefficient
It is the increase in rate when temp is increased by 10 degrees c
When Q10 is exactly 2, rate has doubled, 3= tripled rate
Usually calculated from a graph
Effect of pH
There is an output of pH for each enzyme catalysed reaction
Works fastest at optimal pH- concentration of hydrogen ions determines pH
More ions= lower pH
Differences of x10 doer each pH, e.g. pH 1 to 3= 100 ions
At other pH values, enzymes may function less efficiently to not at all
Pepsin- found in stomach
Chymotrypsin- found in small intestine
Changes in pH can break ionic or hydrogen bonds between R groups, which may result in a shape change of active site, so substrate no longer fits= denatured
Small changes in pH can be reversed as the active site is disrupted slowing the reaction, but its not permanently changed
Large changes of pH will be irreversible
pH and location
Extracellular enzymes may have an optimum pH different to pH 7 depending on location
Salivary amylase- pH 6-8
Pepsin in the stomach- pH 1-2
Trypsin and enterokinase digesting protein in the small intestine work in pH 7-8 (more alkaline than stomach as bile salts are added
Effect of inhibitors
Inhibitors, when added to an enzyme/ substrate mixture, reduce the rate of reaction
There are two types of reversible inhibitor: competitive and non-competitive
Reversible inhibitors: when removed, active sites returns to normal shape
Non-reversible inhibitors: change shape of active sites permanently
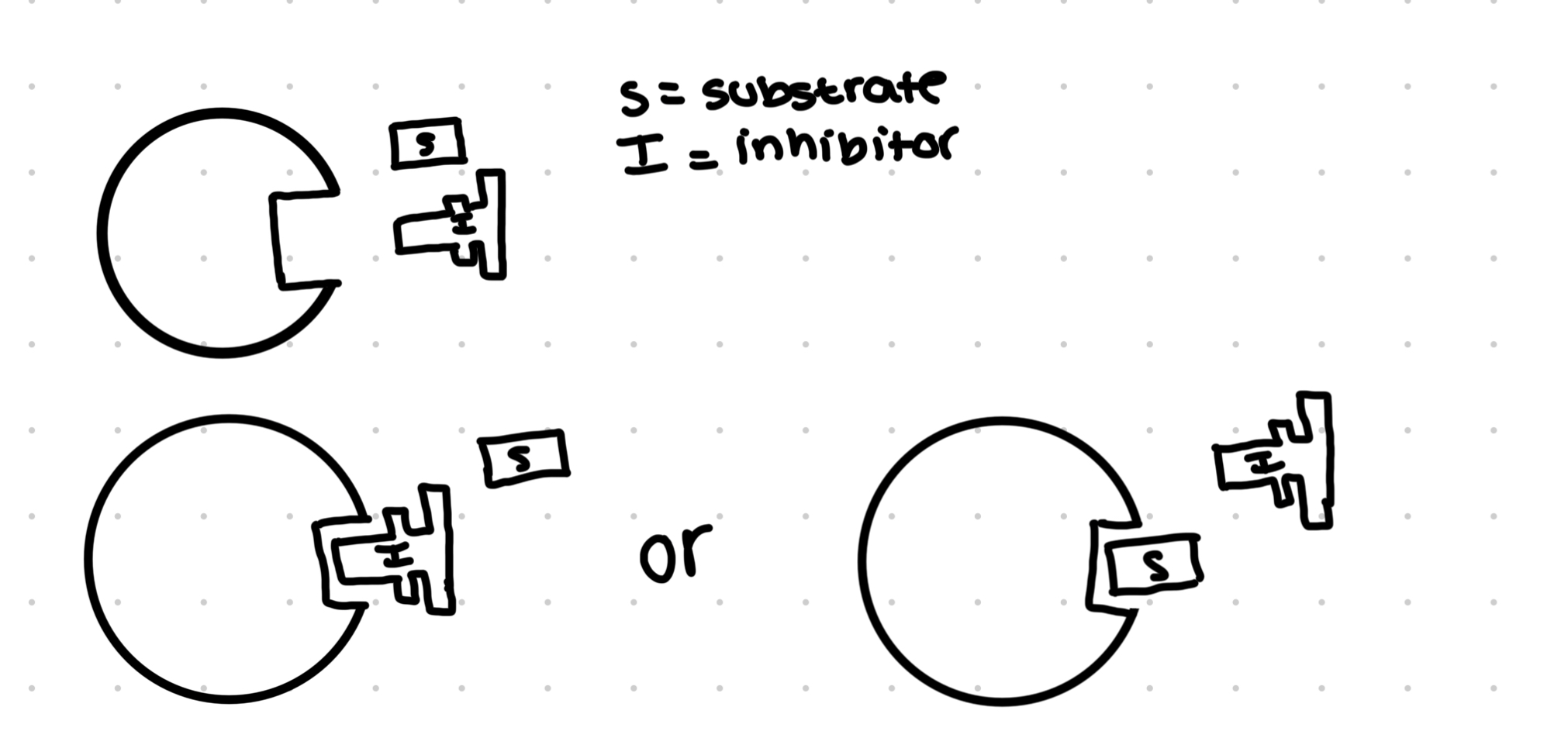
Competitive inhibitors
A competitive inhibitor has the same shape as the substrata molecule
They compete for the same active site
If the inhibitor enters the active site first, it means the substrate wont be able to bind
The relative concentration of inhibitor and substrate will determine the number of active sites occupied by inhibitors and the degree of inhibition
The effect on rate of reaction of higher the more inhibitors
The higher the concentration of substrate, the less inhibition occurs
Inhibition of competitive inhibitors reduces rate of enzyme activity
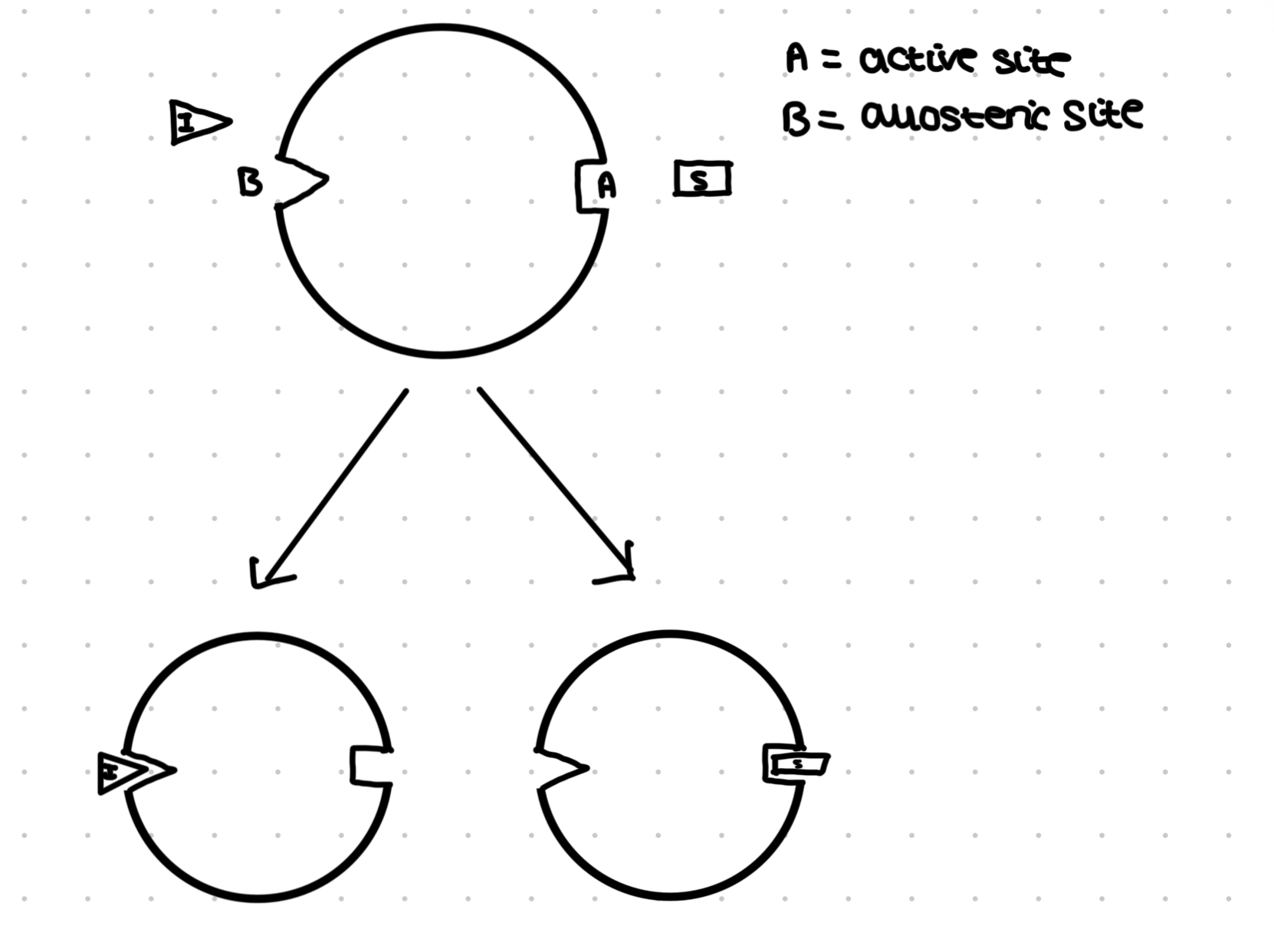
Non- competitive inhibitors
The enzyme has two sites, active site for substrate and allosteric site where the inhibitor may bind
If no inhibitor, the substrate can bind to the active site but the inhibitors can bind to the allosteric site
This causes a change in their 3D shape of their active site pf the enzyme, meaning the substrate can no longer fit (allosteric hindrance)
The amount of substrate and inhibitor determine the rate of reaction
End product inhibition
After a catalysed reaction has completed, product molecules may stay bound to the enzyme, preventing thre enzyme making more of this product
Negative feedback
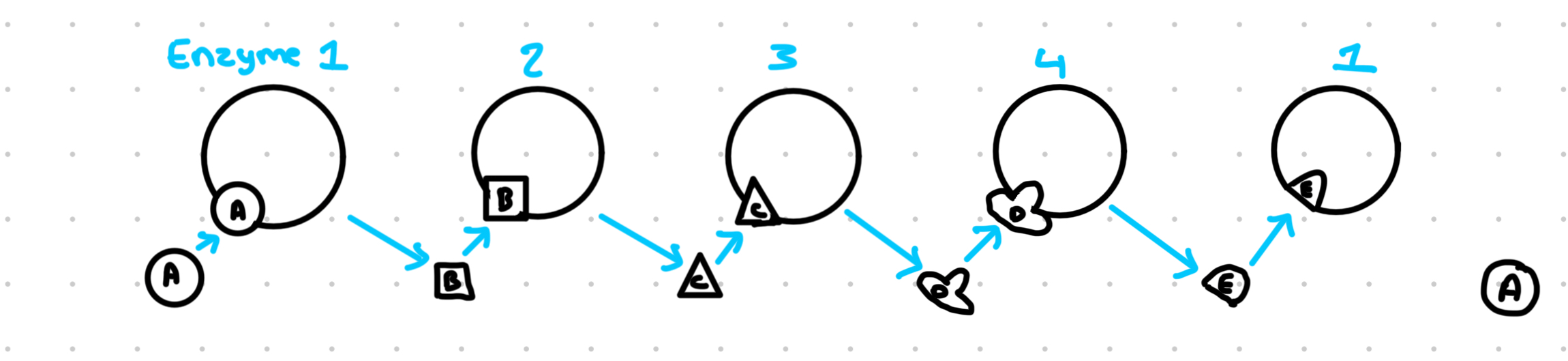
Example of a metabolic pathway:
Substrate A converted to B, B-C, C-D, D-E
End product E can bind to to enzyme 1 and acts as a non-competitive inhibitor, this means that end product E wont build up in the cell, because E is made, it slows down the formation of itself by inhibiting the first enzyme in the sequence
Examples
Enzymes and poisons- cyanide
Enzymes in medicine
Aspirin
Antibiotic resistance
Snake venom
Enzymes and poisons- cyanide
Some poisons work by blocking the action of enzymes
Cyanide, block the action of cytochrome oxidase- an enzyme involved in the final stages of respiration in the mitochondria, cyanide binds H+ there which stops aerobic respiration
It is a non-competitive inhibitor
Only 100-200 mg can make you lose consciousness, this can happen in under 10 seconds
Enzymes in medicine
Can be used to combat infections caused by viruses
E.g. protease inhibitors stop replication of HIV in its tracks as it stops virus being able to make its protective protein coat
This is a competitive inhibitor
Revers transcriptase inhibitors block this enzyme which is essential for viral replication
Aspirin
Combine to enzymes that synthesise prostaglandins- molecules that are important for detecting and repointing to pain, they’re signalling molecules
Cyclooxygenases (COX enzymes) are also responsible for their synthesis and are the sites for other drug use
Antibiotic resistance
Antibiotics are out first, sometimes only, line of bacterial defence
Bacteria have evolved an enzymes that can break down antibiotics such as penicillin- beta lactamase
These bacteria alter then said to be resistant to any bacteria that has a beta lactam ring
Snake venom
Lethal dose of venom and enzyme
Phosphodiesterases are also present in snake venom, affecting functions of the victims hear- some also affect DNA backbones
Also contain acetyl cholinesterase- blocks nerve transmission
Also contain hyaluriclurase- digests connective tissue