Unit 2: Instrumental Methods of Analysis
1/134
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
135 Terms
Instrumental methods of analysis
Methods based on the measurement of various physical and or chemical properties of the analytes using sensing probes or electronic gadgets.
Kinetic methods
An instrumental method characterized by the rate of reaction.
Conductometry
An instrumental method characterized by electrical resistance.
Potentiometry
An instrumental method characterized by electrical potential.
Polarimetry
An instrumental method characterized by the rotation of radiation.
Refractometry
An instrumental method characterized by the refraction of radiation.
Spectrophotometry and photometry
An instrumental method that includes X-ray, UV, Visible, and IR measurements.
Emission spectroscopy
An instrumental method characterized by the emission of radiation.
Raman spectroscopy
An instrumental method characterized by the scattering of radiation.
Chromatography
A group of instrumental procedures used for separation and resolution of closely related compounds.
Potentiometric titration
An electroanalytical technique based on the measurement of the potential of electrochemical cells without drawing appreciable current.
Nernst equation
An equation that relates the potential of an electrode to the concentration of the ion to which it is reversible.
Electrode potential
The potential of an electrode that depends upon the concentration of the ion in accordance with the Nernst equation.
End point of potentiometric titration
The point in the titration where the voltage jump occurs, typically halfway between the jump in voltage.
EMF of the cell
The electromotive force of the cell that depends on the concentration of the electrolytes with which the electrodes are in contact.
Indicator electrode
The electrode used to measure the potential in potentiometric titration.
Reference electrode
The standard electrode used in potentiometric titration for comparison.
pH measurement
A common potentiometric measurement used by manufacturers to assess the acidity of consumer products.
Thermodynamic equilibrium constants
Constants such as Ka, Kb, and Ksp that can be determined using potentiometric measurements.
Electrolyte solution
The solution in which the potential is measured during potentiometric titration.
Consumer products
Products for which manufacturers measure pH using potentiometric methods.
Clinical laboratories
Laboratories that determine blood gases as important indicators of disease states using potentiometric measurements.
Industrial and municipal effluents
Wastewater monitored continuously to determine pH and concentrations of pollutants.
Oceanographers
Scientists who determine carbon dioxide and other related variables in seawater using potentiometric measurements.
Ion-selective membrane electrodes
Electrodes that measure ion concentrations directly from the potential and are free from interferences.
Equivalence point
The point in titration where the quantities of the reacting species are present in equivalent amounts.
End point in potentiometric titration
The point that can be fixed by plotting emf readings against the volume of titrant added.
Cell emf
The electromotive force of the cell that varies gradually but changes abruptly near the end point.
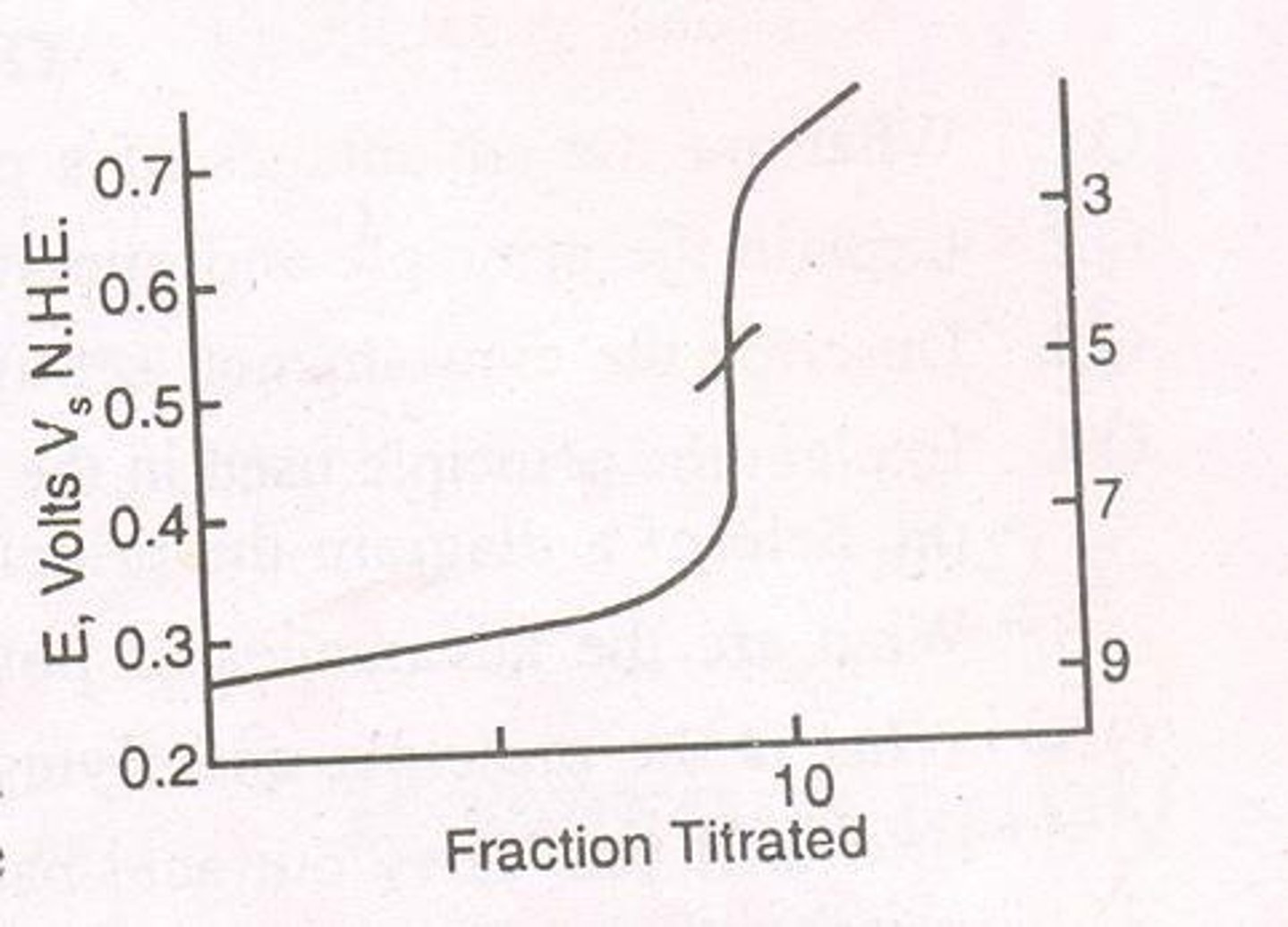
Titration curve
A graph plotting cell emf against the volume of titrant added, showing the relationship during titration.
First derivative method
A technique where the first derivative ΔE/ΔV is plotted against V to locate the maximum at the endpoint.
Second derivative method
A technique where the second derivative Δ2E/ΔV2 is plotted against V, which is zero at the endpoint.
Advantages of potentiometric titration
Includes inexpensive apparatus, easy interpretation of curves, applicability to colored solutions, and analysis of dilute solutions.
Conductometric titration
A method where the end point is determined through conductivity measurements during the titration process.
Henderson-Hasselbalch equation
An equation used to calculate the pH of a buffer mixture of weak acid and its salt.
Buffer solution
A solution consisting of a weak acid and its salt that resists changes in pH.
Conductivity measurements
Measurements taken during conductometric titration to assess the ionic conductivities of the solution.
Titrant
The solution added from the burette during titration to react with the analyte.
Ionic conductivity
The ability of an ion to conduct electricity in a solution, which impacts overall electrolytic conductivity.
Point of inflection
The point on the titration curve corresponding to the maximum rate of change of cell emf for unit volume of titrant added.
Steep portion of the curve
The section of the titration curve where the emf changes rapidly, indicating proximity to the end point.
Burette
A laboratory apparatus used to dispense precise volumes of titrant during titration.
Titration
A quantitative chemical analysis method used to determine the concentration of an identified analyte.
Dilute solutions
Solutions that contain a small amount of solute relative to the solvent, suitable for analysis in titration.
Indicators
Substances used to indicate the end point of a titration, which may interfere with certain titrations.
Bromide and iodide titration
An example of titrating two components in the same solution without interference from indicators.
Conductometric titration
A technique based on the measurement of conductance of the solution.
Titrant
A solution used in titration whose concentration is known and is added to another solution of unknown concentration to determine its concentration.
Analyte
The solution used in titration whose concentration is unknown.
Equivalence Point
The point in conductometric titration at which conductivity undergoes a sudden change.
Conductance
The measure of the ability of a solution to conduct electricity, which depends on the number of free ions, the charge on free ions, and the mobility of the free ions.
Advantages of Conductometric Titration
Conductometric titration does not require indicators, is suitable for colored solutions, provides more accurate results, and can analyze turbid suspensions, weak acids, weak bases, and mixtures of weak and strong acids.
Limitations of Conductometric Titration
Only a few specific redox titrations can be carried out, and it shows less accurate results when the total electrolytic concentration is high in solution.
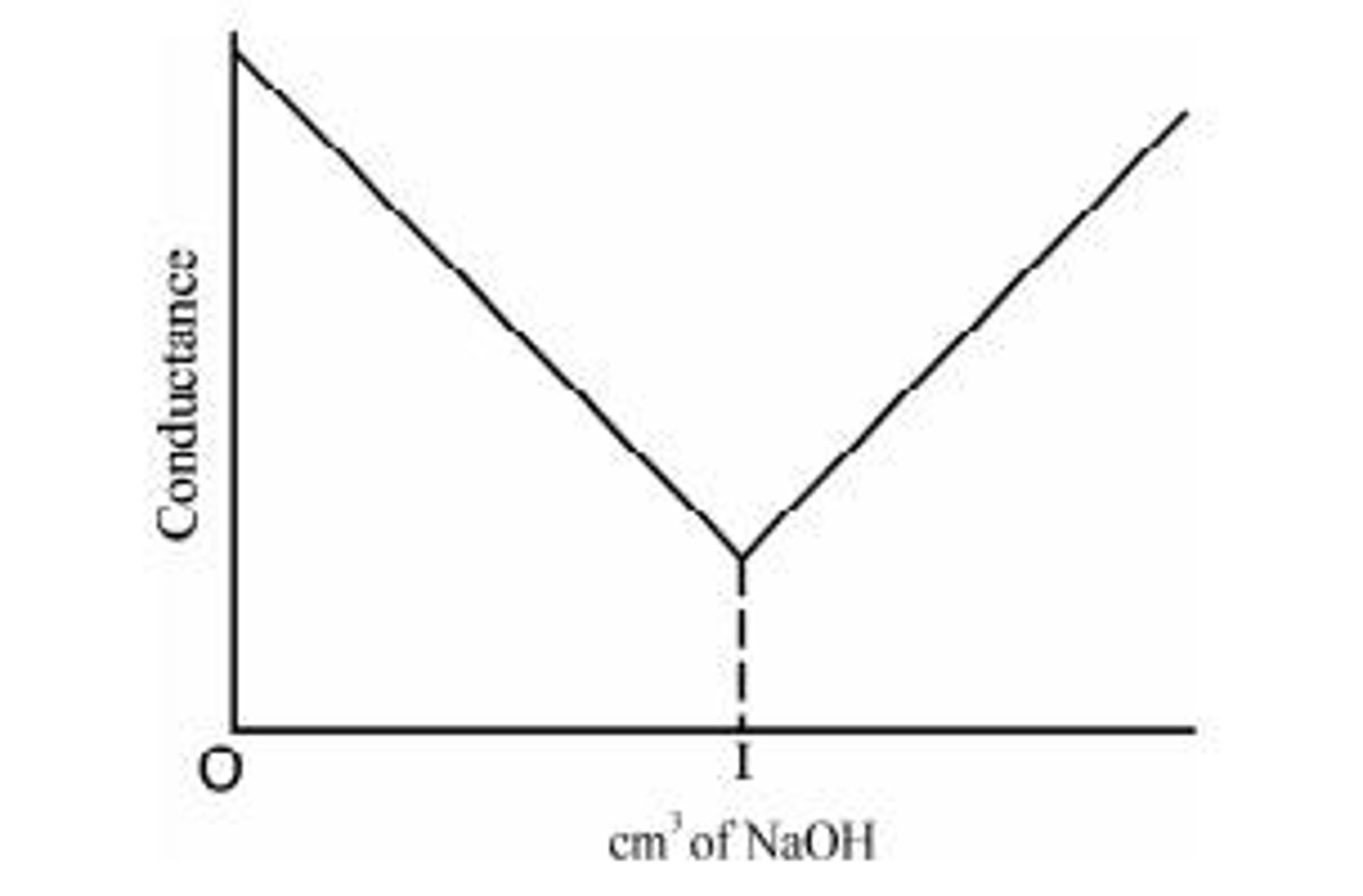
Titration of Strong acid with strong base
An example is the reaction between HCl and NaOH, where conductance falls rapidly due to the replacement of fast-moving hydrogen ions by slow-moving Na+ ions.
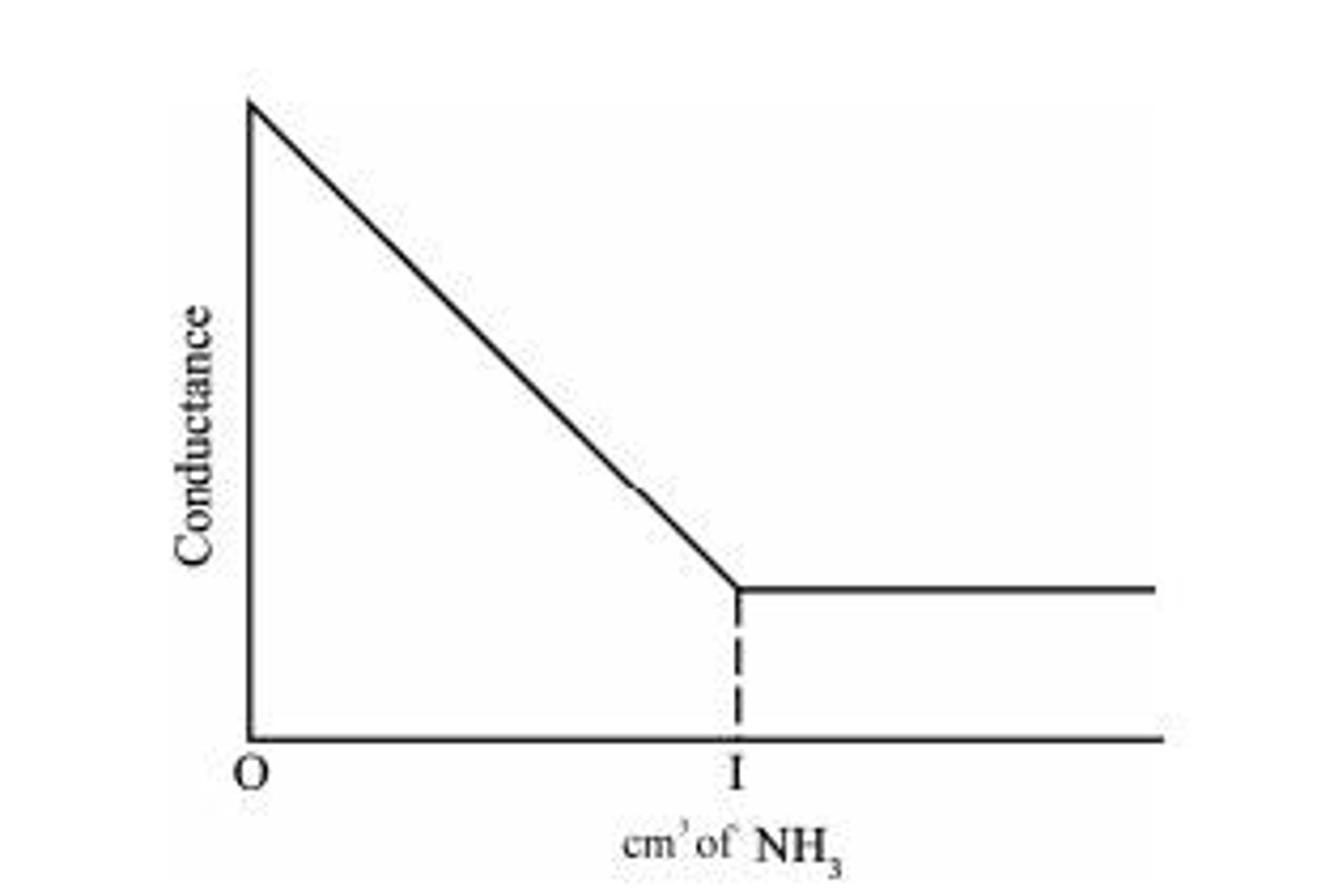
Titration of Strong acid with weak base
An example is the reaction between HCl and NH4OH, where conductance falls rapidly due to the replacement of fast-moving H+ ions by slow-moving NH4+ ions.
Strong acid
An acid that completely dissociates in solution, such as HCl.
Strong base
A base that completely dissociates in solution, such as NaOH.
Weak base
A base that does not completely dissociate in solution, such as NH4OH.
Conductometric titration of HCl vs. NaOH
The conductance decreases until the equivalence point is reached, after which it increases due to the conductivity of OH- ions.
Conductometric titration of HCl vs. NH4OH
The conductance falls rapidly due to the replacement of fast-moving H+ ions by slow-moving NH4+ ions.
Free ions
Ions in solution that are not bound to any molecules and can move freely, contributing to conductivity.
Charge on free ions
The electric charge associated with free ions, which affects their mobility and the overall conductance of the solution.
Mobility of free ions
The ability of free ions to move through the solution, which influences the conductance.
Endpoint in conductometric titration
Determined graphically based on the conductance changes during the titration process.
Graphical determination
A method used in conductometric titration to determine the endpoint by analyzing conductance data plotted on a graph.
Turbid suspensions
Solutions that are not clear due to the presence of suspended particles, which can be analyzed using conductometric titration.
Weak acids
Acids that do not completely dissociate in solution.
Mix of weak and strong acids
A solution containing both weak acids and strong acids, which can be analyzed using conductometric titration.
Conductometric titration
A method to measure the conductivity of a solution during a titration process.
Equivalence point
The point in a titration at which the amount of titrant added is stoichiometrically equivalent to the amount of substance in the sample.
NH4OH
Ammonium hydroxide, a weak base that does not dissociate completely in solution.
Common ion effect
The decrease in solubility of a salt when a common ion is added to the solution.
Conductivity
A measure of a solution's ability to conduct electric current, influenced by the concentration of ions.
Weak acid
An acid that only partially dissociates in solution, such as acetic acid (CH3COOH).
Strong base
A base that completely dissociates in solution, such as sodium hydroxide (NaOH).
Conducting salt
A salt formed from the reaction of an acid and a base that can conduct electricity.
Titration of weak acid with strong base
A titration process where a weak acid is neutralized by a strong base, resulting in a change in conductivity.
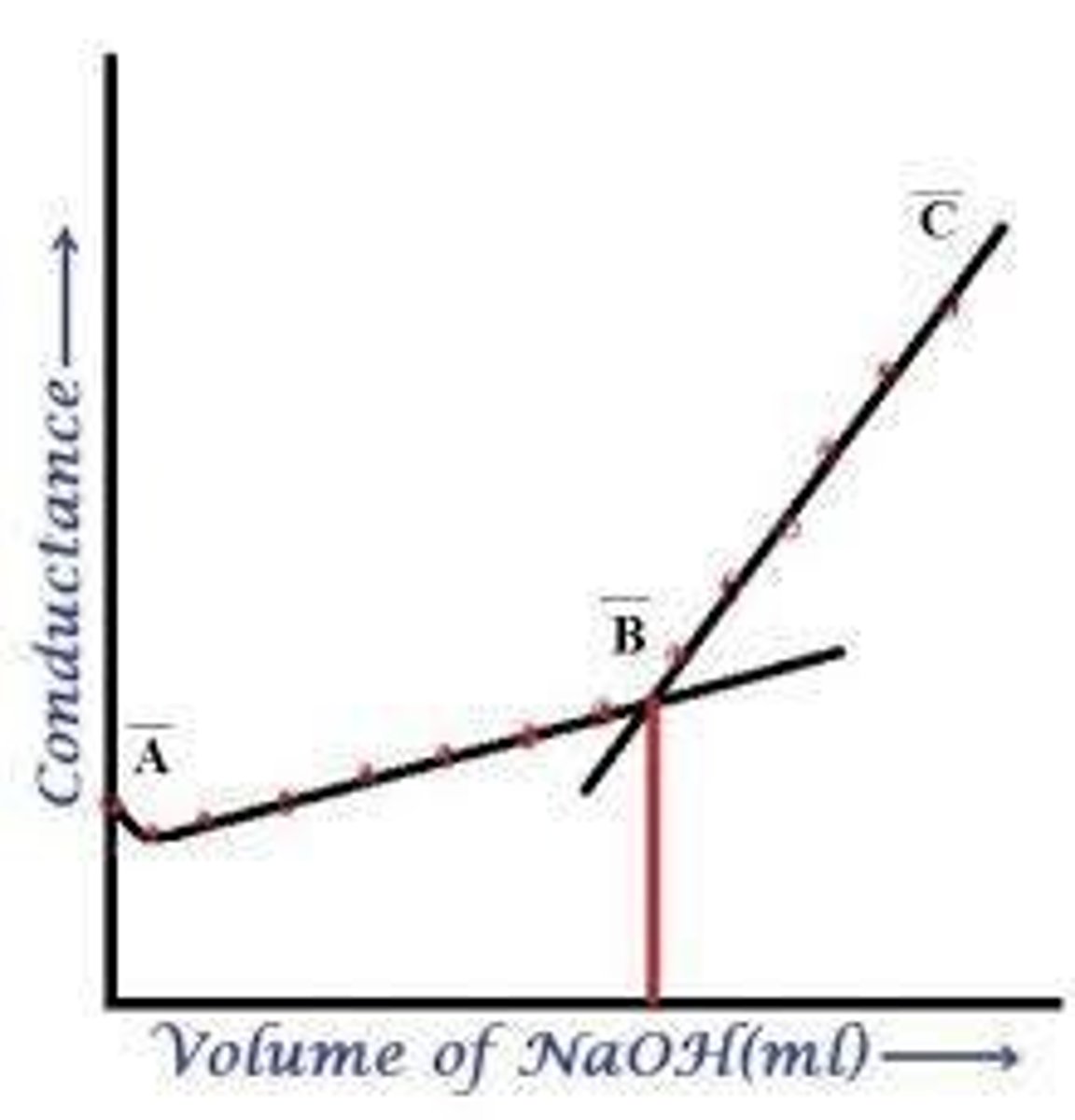
Titration of weak acid with weak base
A titration process where a weak acid is neutralized by a weak base, resulting in less pronounced changes in conductivity.
CH3COOH
Acetic acid, a weak acid that partially dissociates in solution.
NaOH
Sodium hydroxide, a strong base that completely dissociates in solution.
Conductometric titration curve
A graphical representation of conductivity changes during a titration.
Lambert's Law
A principle stating that the rate of decrease in intensity of light is proportional to the intensity of the light and the thickness of the medium.
Intensity of light
The power per unit area carried by a wave, typically measured in watts per square meter.
Thickness of the medium
The distance that light travels through an absorbing medium.
Proportionality constant (k)
A constant that relates the rate of decrease in intensity to the intensity of light and thickness of the medium.
I0
The intensity of the incident light before it passes through the absorbing medium.
It
The intensity of the transmitted light after passing through the absorbing medium.
Exponential decay
A decrease that follows an exponential function, often seen in the intensity of light as it passes through an absorbing medium.
Conductance value
A quantitative measure of a solution's ability to conduct electricity, often changing during titration.
CH3COONH4
Ammonium acetate, a conducting salt formed from the reaction of acetic acid and ammonium hydroxide.
Absorption Coefficient (K)
The reciprocal of the thickness (t, cm) required to reduce the light to 1/10th of its intensity.
Transmittance (T)
The ratio, It/I0, is the fraction of the incident light transmitted by a thickness 't' of the medium.
Opacity
The reciprocal of transmittance.
Absorbance (A)
Given by A = log(I0/It).
Beer's Law
The intensity of a beam of monochromatic light decreases exponentially as the concentration of the absorbing substance increases arithmetically.
Beer-Lambert's Law
The mathematical expression log(It/I0) = aCt.
Molar Absorption Coefficient (ε)
The symbol given to 'a' when C is expressed in mol/l and t in cm.
Specific Absorption Coefficient
Defined as the absorption per unit thickness and unit concentration.
Limitations of Beer-Lambert Law
Linearity is limited by chemical and instrumental factors.
High Concentration Deviations
Deviations in absorptivity coefficients at high concentrations (>0.01M) due to electrostatic interactions.
Light Scattering
Scattering of light due to particulates in the sample.