Topic 4 - Intermolecular Forces and the States of Matter: Types of Bonding
1/9
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
10 Terms
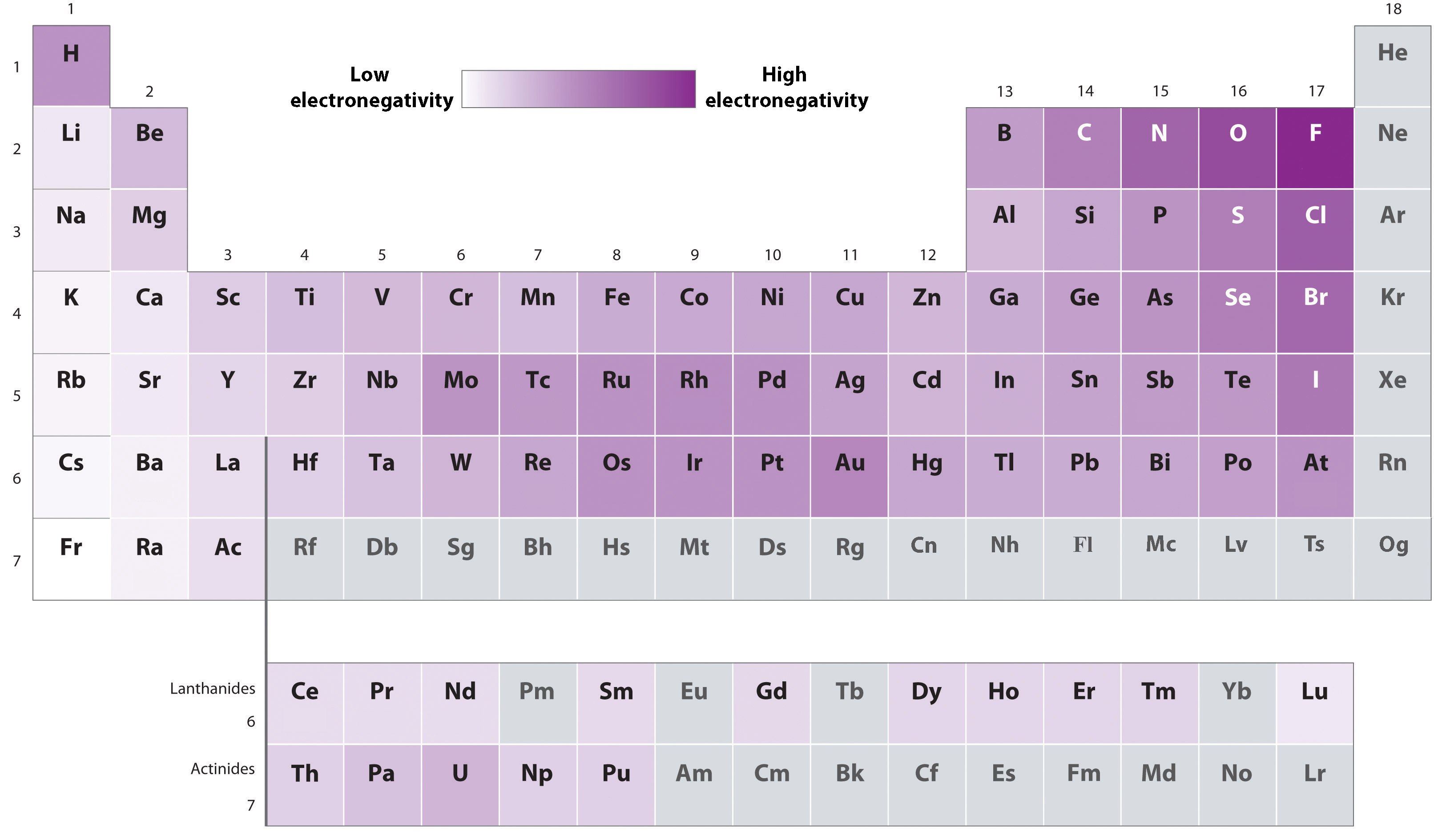
Electronegativity
Electronegativity: Atom’s ability to attract bonding electrons.
Electronegativity varies across the periodic table: Bottom-left of the periodic table tends to be least electronegative, while the top-right tends to be more electronegative.
Difference in electronegativity between two atoms determines bond type:
Larger difference → less equal electron sharing.
Bond Types - Pure Covalent Bonding (Non-polar Covalent)
Definition: Two atoms share one or more pairs of electrons equally.
Occurs when atoms have very small or no difference in electronegativity.
Common between non-metals in Groups 13–17 and hydrogen.
Shared electrons are called bonding electrons and are between nuclei.
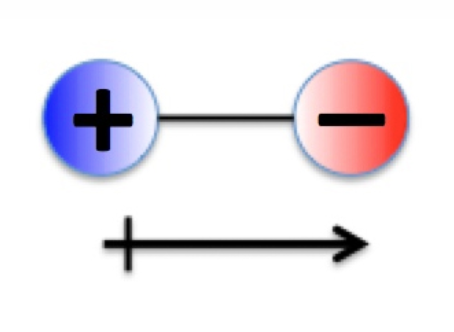
Bond Types - Polar Covalent Bonding
Unequal sharing of bonding electrons.
Both atoms attract electrons strongly, but one is more electronegative.
More electronegative atom pulls electrons closer → partial negative charge; other atom → partial positive charge.
Creates a dipole (equal but opposite charges separated by distance).
Bond Types - Ionic Bonding
Very large electronegativity difference.
One atom (least electronegative) donates electron(s) completely to another (more electronegative).
Forms cation (positive) and anion (negative).
Bond held together by electrostatic attraction.
Usually between metals and non-metals.
Ionic compounds form lattices (not discrete molecules).
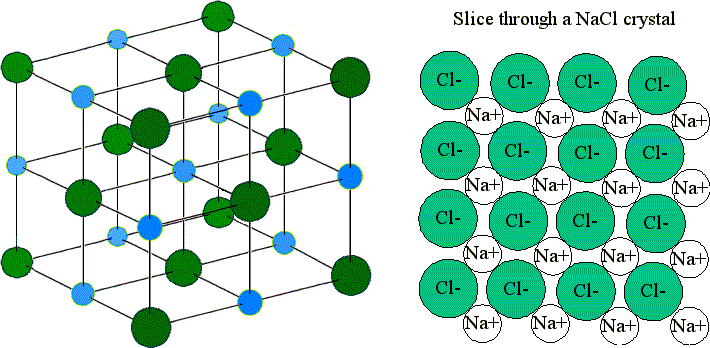
Ionic Lattices
Dense and regular packing of ions which maximizes the interactions between cations and anions, and minimizes the interactions between like-charges.
Bond Types - Metallic Bonding
Chemical bonds between metals, where both atoms have a low electronegativity.
- Equal sharing of bonding electrons in a different way from covalent bonds *not covered in this course
Bonding
Bond type exists on a continuum — no sharp boundaries between categories.
Some sources use Pauling electronegativity values to classify bonds.
All bonds between different elements have some covalent and ionic character.
For this course:
Non-polar = only when atoms are identical (ΔEN = 0).
Other small electronegativity differences treated as polar covalent.
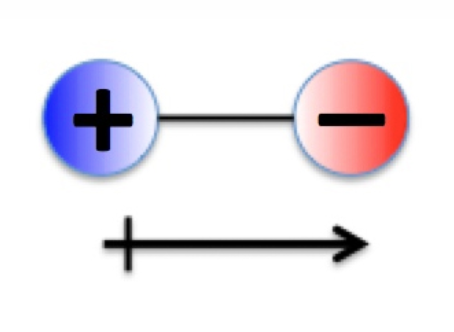
Bond Types - Polar Covalent Bonding: Dipoles
Polar bonds have dipoles (positive and negative ends).
Dipoles represented as vectors:
Tail with cross = positive end.
Arrowhead = negative end.
Vector representation helps analyse dipole direction in 3D space.
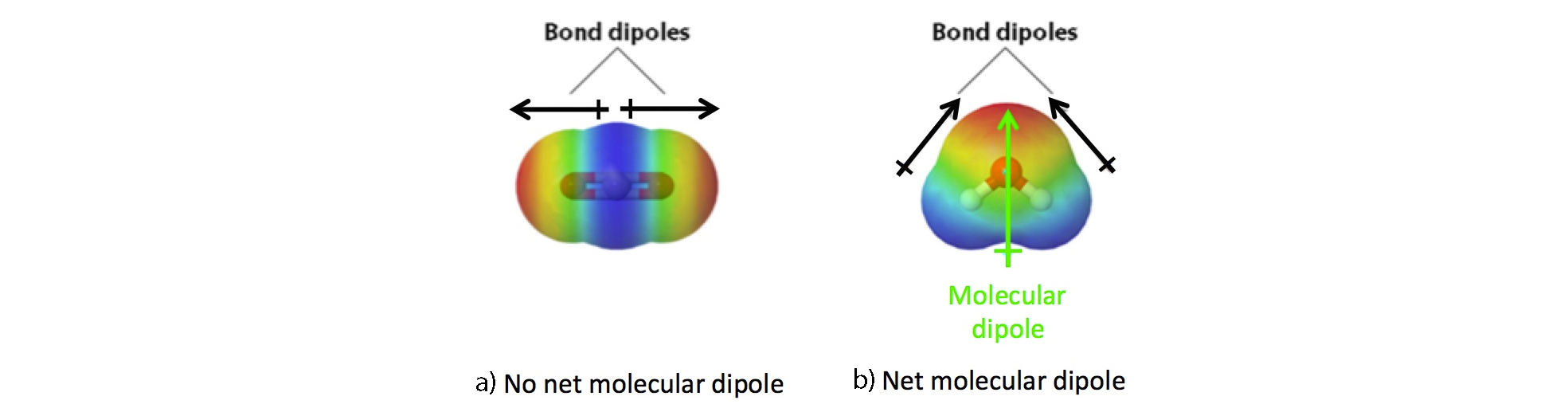
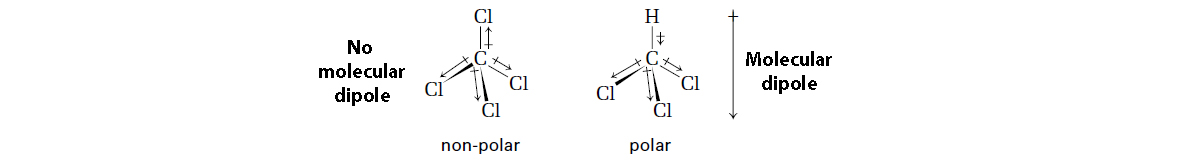
Molecular Polarity
While polarity applies to specific bonds, it can also be applied to an entire molecule, considering all the bonds in the molecule.
Polarity of a molecule = size of overall net dipole.
Two conditions for a molecule to be polar:
Polar covalent bonds are present.
Molecule is asymmetrical (not linear and opposite from each other, but bent and/or going in the same direction)→ dipoles do not cancel.
Notes on C–H Bonds
Hydrocarbons:
C–H bond is weakly polar, but symmetrical arrangements → no net dipole → non-polar.
C–H treated as weakly polar; can be approximated as non-polar for net dipole determination.
Molecules with Polar Bonds + Long Hydrocarbon Chains:
Can have both polar and non-polar regions.
Example: Pentanol - OH group polar, long C–H chain non-polar.
Long non-polar chain influences physical properties.
Longer hydrocarbon chain = more non-polar behaviour.