Topic 1: Inorganic Chemistry I - Chelate Effect
1/20
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
21 Terms
Effective Nuclear Charge (Zeff) and Ionic Size
As one moves across a row of transition metals (left to right), the nuclear charge increases and electrons are added to the same shell, causing ion sizes to decrease and Zeff to increase, which influences stability constants.
Gibbs Energy (ΔG)
ΔG = ΔH - TΔS = -RTlnK
Where:
ΔG - Gibbs free energy: measures the spontaneity of a reaction. ΔG < 0 means the reaction is spontaneous, ΔG > 0 means the reaction is not spontaneous
ΔH - Enthalpy change: the heat absorbed or released during a reaction. ΔH < 0 means the reaction is exothermic, ΔH > 0 means the reaction is endothermic
T - Temperature: measured in kelvins (K). Higher temperatures can affect reaction spontaneity
ΔS - Entropy change: the change in disorder or randomness of the system. ΔS > 0 means increased disorder, ΔS < 0 means decreased disorder
R - Universal gas constant: equal to 8.314 J·mol⁻¹·K⁻¹
K - Equilibrium constant: indicates the extent to which a reaction proceeds toward products
ln - Natural logarithm: used to relate Gibbs energy to the equilibrium constant through a logarithmic relationship
Stability Constant (β)
The stability constant β is the equilibrium constant for the formation of a complex in solution
Also called the formation constant (Kf).
Cumulative constant: β often represents the complete complexation process even when complexes form in steps
Stability Prediction
Larger β (or log β) means a more stable complex.
Thermodynamic Nature of β
β is thermodynamic—it indicates equilibrium composition but not the speed of formation (kinetics).
Dissociation Constant (Kd)
Kd = 1/β.
The smaller the Kd, the more stable the complex.
Stability Constant Formula
For the ligand displacement reaction: M(H2O)xⁿ⁺ + yL ⇌ MLyⁿ⁺ + xH2O
β = [MLyⁿ⁺] / ([L]y [M(H2O)xⁿ⁺]).
Water is omitted from the equilibrium expression because its concentration is effectively constant in aqueous solution.
Ligand Displacement Reactions
A new ligand forms a more stable complex, displacing the existing ligand.
Examples:
Cyanide and Iron: [Fe(H2O)6]²⁺ + 6CN⁻ ⇌ [Fe(CN)6]⁴⁻ + 6H2O.
Nickel and Copper: [Cu(H2O)6]²⁺ + 4NH3 → [Cu(NH3)4(H2O)2]²⁺.
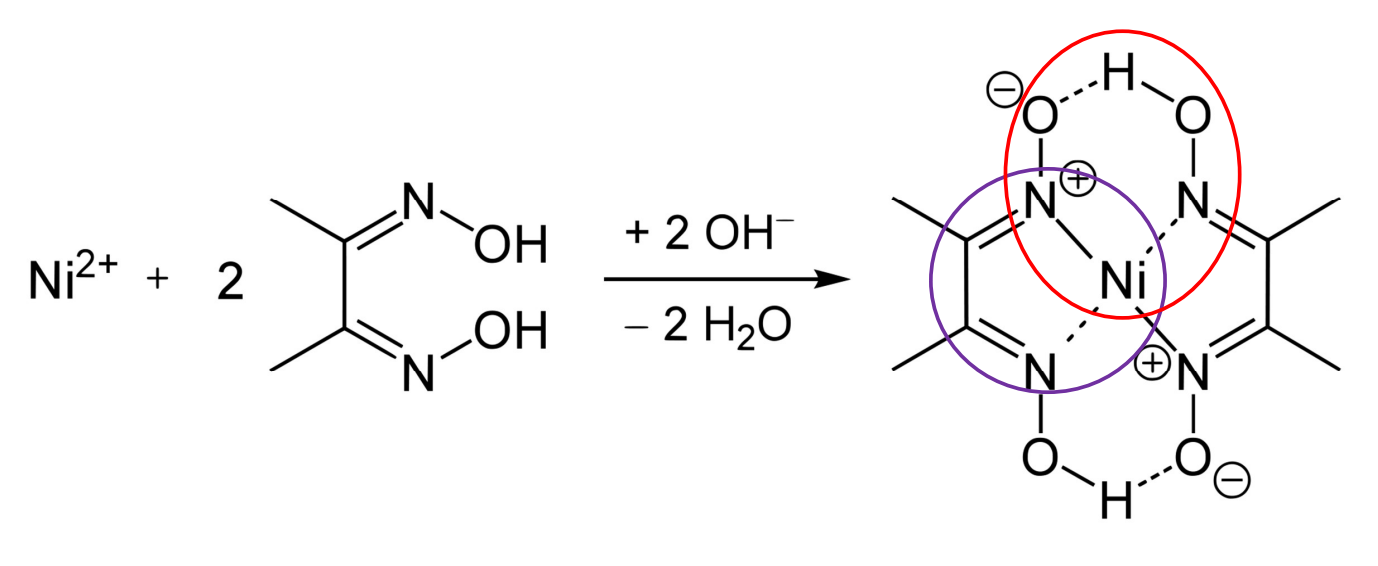
Chelate Effect
Complexes with multidentate ligands are more stable than those with monodentate ligands because they form ring structures.
Chelate Geometry
Chelates form 5- or 6-membered rings, which are usually most stable.
Thermodynamic Origin of Chelate Effect
The chelate effect is driven by entropy (ΔS):
From the ligand displacement reaction, the amount of discrete molecules released increases
E.g: [Ni(NH3)6]2+ + 3 en ⇌ [Ni(en)3]2+ + 6 NH3, the reaction starts with 3 discrete molecules, but ends by releasing 6 discrete molecules
More particles → more disorder → more entropy (ΔS) → thermodynamically favoured → more stability
From the Gibbs free energy equation, a greater entropy leads to greater spontaneity, and hence more stability
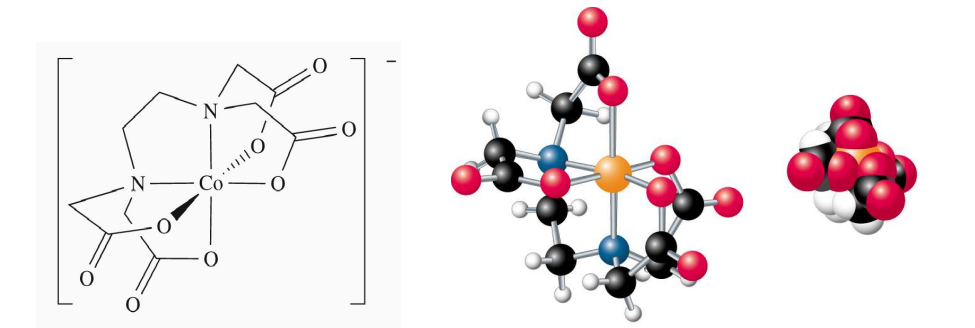
Optimum Chelate Effect (EDTA)
Hexadentate ligands like EDTA⁴⁻ create highly stable complexes, displacing multiple water molecules and increasing entropy.
When one EDTA⁴⁻ binds, 6 water molecules are released (2 → 7 particles), giving a large entropy gain.
Factors Affecting β: Charge-to-Radius Ratio
Higher charge and smaller radius increase β, strengthening metal-ligand attraction.
Example:
Higher Charge: Fe²⁺ → Fe³⁺
Smaller Radius: Across transition metals with same charge, Fe²⁺ < Co²⁺ < Ni²⁺ < Cu²⁺.
Factors Affecting β: Chelation
Increased chelation raises β because it releases more discrete particles (higher entropy) and thus greater stability.
Complex Formation and Solubility
Stable complex formation with the addition of a ligand increases the solubility of sparingly soluble salts by reducing [Mn⁺].
Complex Formation and Solubility: Solubility Equilibrium
Sparingly Soluble Metal Salt: MXn (s) ⇌ Mn+ (aq) + nX ⁻(aq) - Equilibrium constant: Ksp
*(aq) suggests Mn+ is surrounded by water ligands
Complex Formation and Solubility: Complex Formation
Complex Formation with the Addition of a Ligand: Mn+ (aq) + yL (aq) ⇌ MLy (aq) - Equilibrium Constant: β
Forming complexes lowers [Mn+] → shifting Ksp right → and dissolving more MXn (s).
Beer-Lambert Law Principle
Absorbance of light (A) is directly proportional to the concentration (c) of a colored solution.
Beer-Lambert Mathematical Expression
A = log10(Io/I) = ε·c·L.
Beer-Lambert Variables
A = absorbance
Io = incident light
I = transmitted light
ε = molar absorption coefficient (L·mol⁻¹·cm⁻¹)
c = concentration
L = path length (cm).
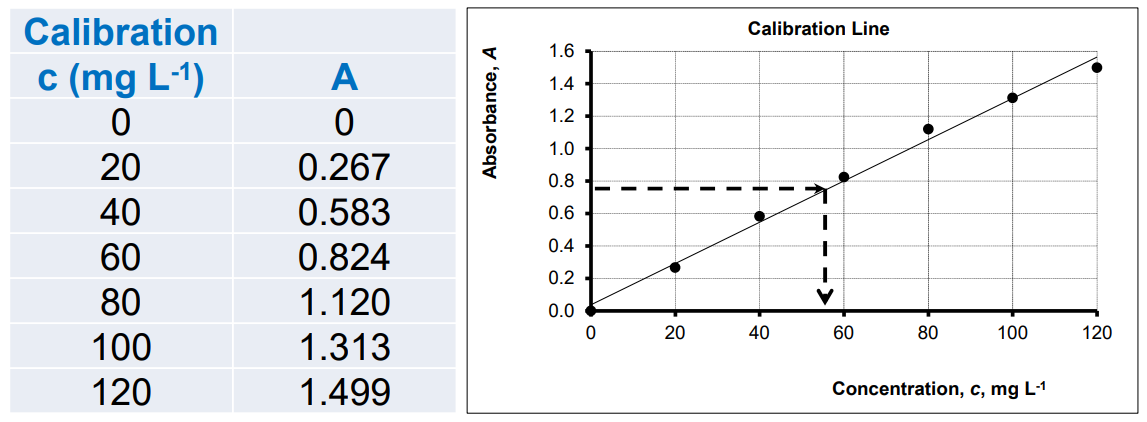
Calibration Curve
A plot of absorbance (A) vs concentration (c) gives a straight line; used to determine unknown concentrations.