Chapter 3 - enzymes only.
1/39
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
40 Terms
Independent variable
the factor that is being deliberately manipulated to determine how it affects the results
Dependent variable
factor that changes due to the changes being made to the dependent variable
Controlled variable
factors kept the same for both the control and experimental groups
Control groups and experimental groups
Experimental Group:
Receives independent variable to see if it causes a change.
Control Group:
does not receives dependent variable and is used for comparison to see if the treatment caused the change.
Validity
The extent to which an experiment tests what it is supposed to test
how to ensure validity
Identify and control for confounding variables (external factors that could interfere with results).
Design the study with a clear distinction between the experimental and control groups. Ensure they are similar in all characteristics, except for independent variable
Use valid instruments and techniques for measuring variables that are accurate and relevant to your hypothesis.
reliability
the extent to which an experiment gives the same result each time it is performed
How to increase reliability
Perform multiple trials and compare the outcomes. If results are consistent, reliability is ensured.
Write a detailed experimental protocol that everyone follows to ensure consistency.
Use calibrated instruments and fixed settings (e.g., time of day for testing, environmental conditions).
Train all researchers thoroughly on the experimental procedure to ensure consistency in how measurements are taken and interpreted. (inter-rater reliability
Accuracy
The extent to which the measurement are correct
How to ensure accuracy
Regularly calibrate instruments and check for maintenance to ensure they measure accurately.
Double-check measurements, and if possible, have another person verify the results.
Use automated data collection methods if available (e.g., using digital thermometers instead of manually recording temperatures).
Keep detailed logs of every aspect of the experiment (e.g., time of testing, environmental conditions) to minimize accidental data distortion.
cross verification (Use more than one method to measure the same thing to ensure accuracy.)
human error + examples + how to avoid
simply a mistake
incorrectly reading the scale
spilling liquid before measuring volume
mistake in calculations
not part of experimental error, should be avoidable with sufficient care and checking
Double-check measurements
Use automated tools
Use consistent units and symbols
random errors
unpredictable errors that can occur in all experiments, because no measurement can be made with absolute precision
stopwatch might stop a little early or late (not a human error, js a limitation of the timing procedure)
Temperature or humidity changes might slightly affect measurements
how to avoid
Repeat measurements
Use precise instruments
Control environmental factors
Systematic erros
occur because of the way an experiment is designed or due to problems with equipment
examples
measuring from the soil instead of the base of the stem, your results will always be off in the same direction.
A thermometer that always reads 2°C higher than the actual temperature
how to avoid
Calibrate instruments:
Follow proper measurement techniques
Cross-check with different instruments
metabolism
all the chemical reactions that take place in cells and therefore organisms
concerned with maintaining a balance between energy release and energy utilisation
two types of metabolism and what they do
anabolism → type of reaction where small molecules built up into larger ones such as protein synthesis. require energy
catabolism → type of reaction where larger molecules are broken down into smaller ones such as digestion. release energy
nutrient + 6 types
any substance in food that is used for growth, repair, or maintaining a body; that is, any substance required for metabolism
water
carbohydrates
lipids
proteins
minerals
vitamins
organic compounds
molecules that have a carbon chain
also contain number of hydrogen atoms and can include atoms of sulfur, oxygen and nitrogen
carbohydrates: what are they, types, what they contain
main source of energy for cells
glucose (a simple sugar) is used in cellular respiration to release energy
starch (complex carb) broken down into simple sugars
contain atoms of carbon, hydrogen and oxygen (twice as many hydrogen atoms and oxygen atoms)
monosaccharides
disaccharides
polysaccharides
Monosaccharides, disaccharides and polysaccharides
simple sugars = monosaccharides (glucose, fructose, galactose)
Two simple sugar molecules bonded togethe = disaccharides (sucrose, maltose, lactose)
larger carbohydrate molecules formed when simple sugars join together = polysaccharides (glycogen, cellulose, starch)
lipids; what are they, use in cellular respiration,
Large organic molecules made up of fatty acids and glycerol
broken down to fatty acids and glycerol
e.g phospholipds
consists of one molecule of glycerol and one, two or three fatty acid molecules
most common fat is triglyceride (stored in body, consists of glycerol and three fatty acid molecules
Proteins
organic compounds that are made up of amino acids
most important in metabolism: enzymes
amino acid: molecule that contains amino group and carboxylic group
peptide bond: two amino acid bonds join together, releasing water molecule
dipeptides: shorter lengths of amino acids with two amino acids joined
polypeptides: 10+ amino acids
inorganic compounds
not based on carbon chain
most do not contain carbon atoms at all, but those that do (carbon dioxide) only in small amounts
water, minerals and vitamins
waters use in metabolism
fluid in which other substances are dissolved
some of cells chemical reactions occur in water
some water molecules take part in reaction
mineral use in metabolism
may be part of enzymes
may function as cofactors
may be part of substances such as ATP that are involved in metabolism
vitamin use in metabolism
act as coenzymes for many reactions
activation energy
the energy needed to break the bonds of the reacting particles in a chemical reaction; the energy needed to start a chemical reaction
any given temp, there is certain proportion of particles that have enough energy to satisfy activation energy. proportion will increase when temperature increases
catalysts, how they affect activation energy
chemicals that are able to decrease the amount of energy needed in activiation energy
This means that the activation energy will be lower and more particles will have enough energy to react, making the reaction happen at a faster rate.
enzymes
Enzymes are biological catalysts that are able to speed up chemical reactions by lowering the activation energy.
They are not consumed or altered in the reaction.
substrate
molecules on which an enzyme acts on
enzyme will only combine with one particular substrate and is therefore involved in only one specific reaction. this is because the enzyme and substrate have complementary characterstics (shape and structure)
active site
part of enzyme molecule that combines with the substrate
when combined, called enzyme substrate complex
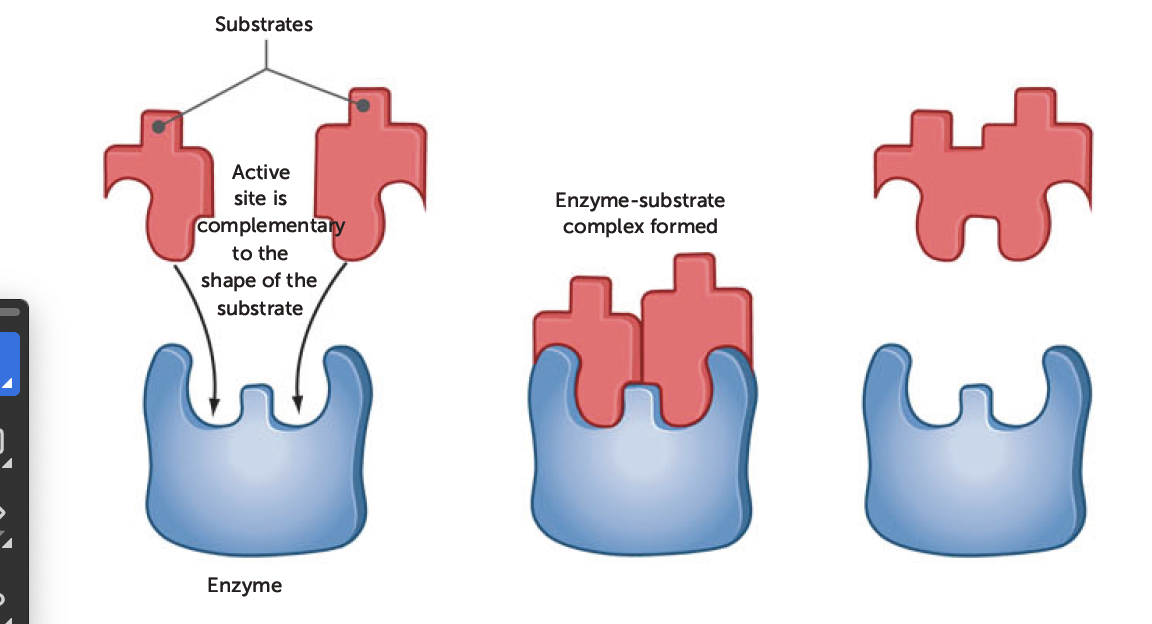
Lock and Key model
shape of enzyme (key) is always complementary to shape of substrate (lock)
therefore, two will fit exactly to form enzyme-substrate complex
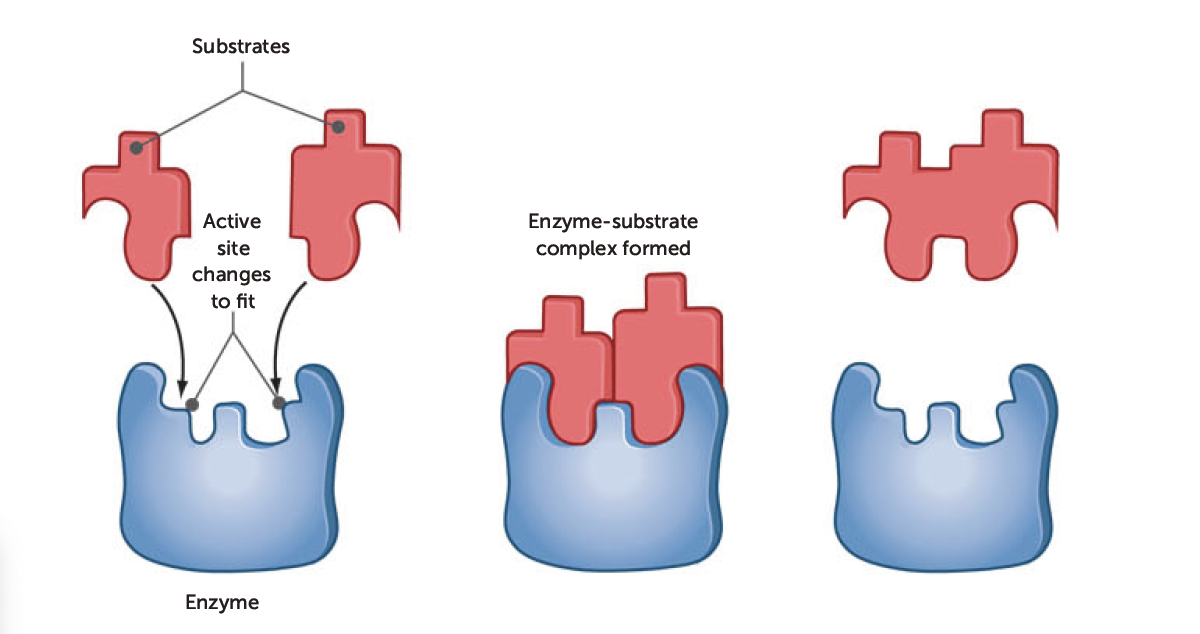
induced-fit model
when enzyme and substrate join, they form weak bonds that cause the shape of the enzyme to change, creating complementary shapes
factors affecting enzyme activity (7)
concentration of enzyme
concentration fo substrate
continual removal of products of reaction
temperature
pH level of medium in which reaction is taking place
co-factors and co-enzymes
enzyme inhibitors
concentration of enzyme (3)
higher concentration of enzyme = faster rate of reaction
why? more enzyme molecules to influence reactants
by regulating type and number of enzymes present, body can control which reactions to occur and the rate at which they proceed
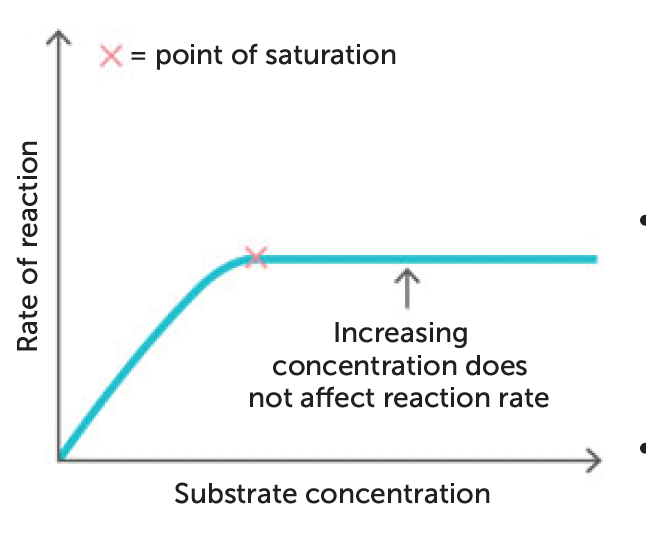
concentration of substrate
higher substrate = increased rate of reaction
why? → more substrate molecules coming into contact with enzyme molecules
but? → increasing substrate beyond a certain concentration will cease to have effect because active sites on enzyme will be fully occupied
continual removal of products of reaction
must be removed continually otherwise rate of reaction will slow due to it becoming more difficult for substrate molecules to make contact with enzyme molecules
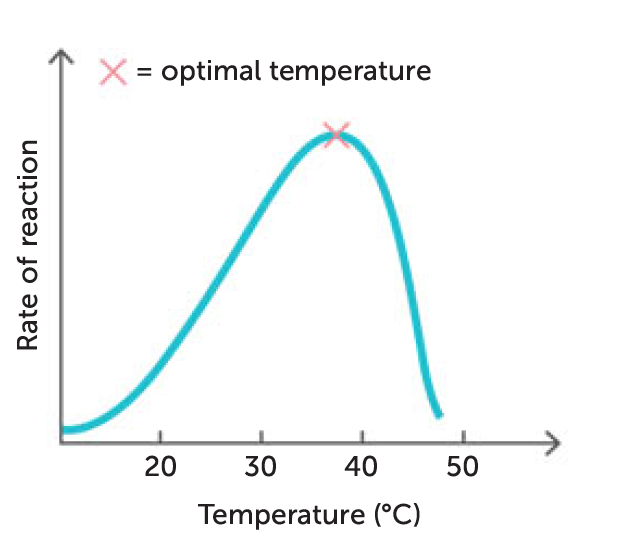
temperature
rate of most chemical reactions increases as temp increases
true for most enzymes but only within limited temp range
because enzymes are proteins, beyond 45 - 50 degrees, structure changes and become denatured
temperature at which an enzyme works best is optimum temperature (for most enzymes in the body this is 30-40 degrees)
pH of the medium in which a reaction is taking place
very sensitive to pH of medium in which a reaction is taking place
each enzyme has optimum pH at which it will work most effectively
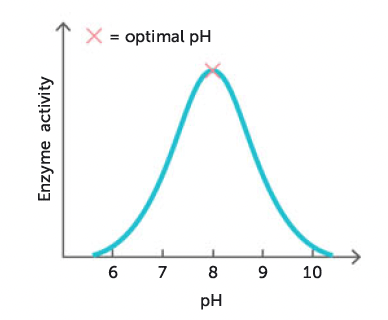
co-factors
certain ions and non-protein molecules needed before enzymes catalyse a reaction
change shape of active site so that enzymes can combine with substrate
no co-factor = intact enzyme molecule, but unable to function
co-enzymes
organic, non-protein molecules that help enzymes catalyze reactions by transferring chemical groups between substrates
no co-enzyme = Failure to bind to the substrate properly, inablity to carry out the chemical reaction needed for the metabolic process
many vitamins, NAD+