Galvanic Cells
1/77
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
78 Terms
Redox Reaction:
A chemical reaction involving the transfer of electrons from one chemical species to another
Are redox reactions exothermic or endothermic?
Exothermic, energy is released during the transfer of electrons in electrical energy or heat energy.
Oxidising agent:
Causes another species to lose electrons, is reduced
Reducing agent:
Causes another species to get reduced, is oxidised.
Oxidation number of oxygen in a compound:
-2, except in peroxides when it is -1
Oxidation number of hydrogen in a compound:
+1, except in NaH when it is -1
When oxidation occurs, oxidation numbers increases/decreases:
Increases
When reduction occurs, oxidation number increases/decreases:
Decreases
KOHES
K - balance key elements
O - balance oxygens by adding H2O
H - Balance hydrogens by adding H+ ions
E - Balance charges by adding electrons
S - Add states
If basic solution:
Add hydroxide ions (OH-) equal to the number of H+ ions to both sides, so all H+ ions convert to H2O (OH- + H+ ------> H2O)
Order of conjugate redox pairs:
oxidising agent/reduced form
Check this
What is an electrochemical cell?
An electrocheical cell is a device in which chemical energy is converted to electrical energy or vise versa.
What is a galvanic cell?
A type of electrochemical cell in which chemical energy is converted to electrical energy in a spontaneous, non-direct redox reaction.
Electrode:
A solid conductor in a half cell at which oxidation or reduction occurs.
What is generally involved in a galvanic cell:
-Salt bridge
-Oxidant and reductant solutions
-Electrodes
-Wire/conductor/external circuit
Draw a general galvanic cell containing Copper and silver half cells, calculate voltage as well:
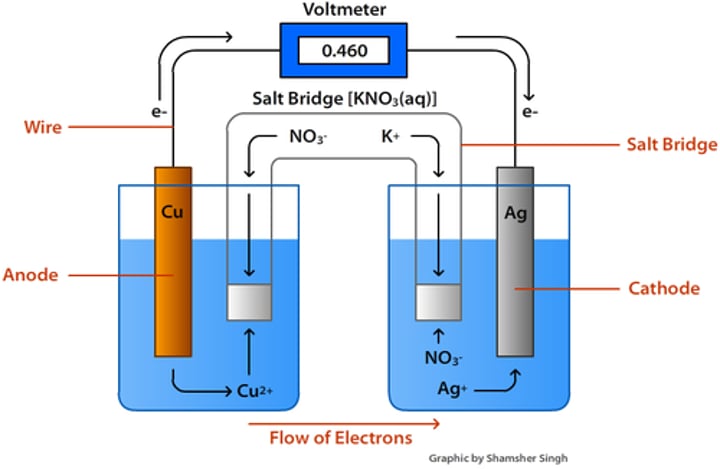
Sign for voltage:
+ or -
Purpose of salt bridge:
-Ions move to balance charges with half cells
-Complete the internal circuit
Features of electrodes:
-Solid
-May need to be inert
-Conductive
-Large surface area to maximise the current
-May contain catalyst to speed up reaction
Anode Polarity
Negative
Cathode Polarity
Positive
What are some common inert electrodes?
-Platinum
-Graphite
-Gold
How is the potential difference between 2 half cells calculated?
Eo oxidising agent - Eo Reducing agent
What is the Eo value a measure of?
The Eo value indicates the tendency of a half cell to undergo reduction
What are the standard conditions?
-25oC
-1 bar
-1 M
What is the standard half cell used as a reference to calculate the Eo value of others half cells?
The H+/H2 standard hydrogen half cell
The strongest oxidising agent is found:
Top left of electrochemical series
The strongest reducing agent is found:
Bottom right of electrochemical series
Limitations of the electrochemical series:
1. The values in the electrochemical series are only valid at standard conditions. The values and order can change when concentration, temperature or pressure change.
2. The electrochemical series provides no information about the rate of reaction.
What is a direct redox reaction?
When the reactants react in direct contact with one another.
What is the energy transformation?
Chemical energy is converted into thermal energy.
Primary Cell:
Galvanic cell that cannot be recharged as products migrate away from contact with the electrodes or are consumed by side reactions. Can't be reused
Secondary Cells:
A electrochemical cell that acts as a galvanic cell when discharging, converting chemical to electrical, but operates as an electrolytic cell when recharging, converting electrical to chemical.
The products that formed during the discharge reaction remain in contact with the electrodes, allowing it to be recharged.
Can be reused.
Do you need a higher/lower/same voltage when recharging a secondary cell and why?
Slight higher. Since a non-spontaneous reaction is being forced, more energy is required.
Why is lithium commonly used in galvanic cells?
Has a high energy density:
-highly reactive
-Light weight
Properties of separators in primary and secondary cells:
-Unreactive with the species present so unwaned side reactions don't occur.
-Structurally stable so that good separation is maintained.
-Can allow some ions to flow through (porous)
Battery:
A combination of cells connected in series.
Battery Life:
Number of discharge-recharge cycles before a battery becomes unusable
Battery capacity:
The amount of charge available
What is the ideal temperature for batteries in terms of battery life?
In terms of modern batteries, room temperature as they are made to work optimally at these temperatues.
Factors that influence battery life over time, i.e. degrade batteries
-Loss of active materials due to side reactions occurring, consuming active materials.
-Progressive conversion of small crystals of active material to larger crystals, increasing resistance to current flow (less surface area)
-Formatin of other chemicals in side reactions impeding cell functioning.
-Decrease in contact of electrolye with electrodes, hindering balancing of charge.
-Temperature
What happens to battery life as temperature increases and why?
Battery life decreases beacse the rate at which side reactions are occurring is faster, causing more active material to be used up and battery function to be impeded.
What happens to battery capacity as temperature increases and why?
Battery capacity increases because the high rate of reaction allowing electron transfer to occur faster.
Fuel Cells:
-A type of galvanic cell that does not store energy which requires a constant supply of reactants.
Features of a fuel cell:
-Contains 2 compartments: the fuel at the anode and oxygen at the cathode.
-Contains 2 porous electrodes, allowing reactants to diffuse through them to react with ions in the electrolyte. Electrodes are in contact with electrolyte.
-Electrolyte that carries ions from one electrode to the other.
-Catalysts to enhance the rate of reaction and the current that can be produced.
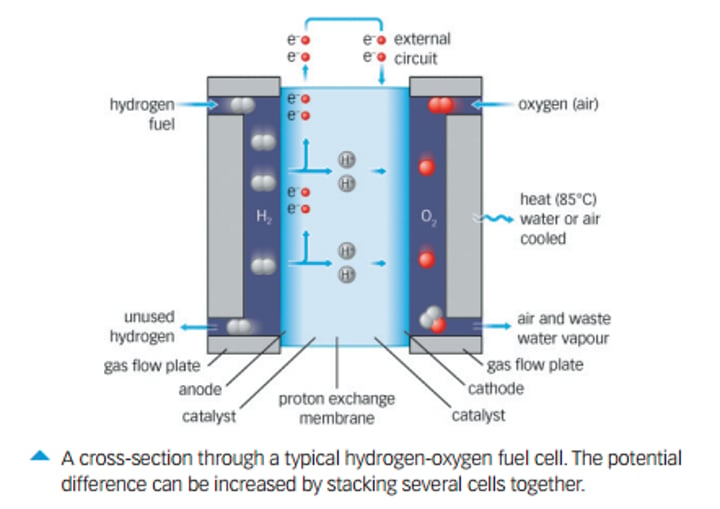
Does a fuel cell contain half cells?
Yes, despite being in one vessel.
Most common electrolyte:
KNO3(aq), potassium nitrate.
Properties of electrodes in fuel cells:
-Porous so allows reactants to diffuse through them and react with ions in electrolyte.
-Conducts charge, allowing electrons to flow through them
-Contain catalysts which can speed up the reaction rate
Current:
The amount of charge passing through a given point per unit of time.
Examples of catalysts:
-platinum
-nickel
-nano material
Energy transformations in fuel cell vs petrol engine:
Petrol Engine: Chemical ---> thermal ----> mechanical
Fuel Cell: Chemical ------> electrical -----> mechanical
Energy transformations in fuel cell release less energy, thus making it more energy efficient
Advantages Fuel Cells:
-40-60% energy efficiency, compared to 30-40% for thermal power stations and 25-30% for car engines.
-Environmentally friendly as H2 fuel cells produce no greenhouse gases.
-Continual production of electricity whilst supplied with fuel
-On site electricity production (no need to be connected to supply grid)
Disadvantages of Fuel Cells:
-Requires a constant supply of fuel
-Currently expensive
-Produce DC current, whereas homes and industries require AC current, some need to convert power.
-Comprehensive storage and safety for hydrogen gas.
Do hydrogen fuel cells produce greenhouse gases?
Yes - the oxygen is reduced to water, and water is a greenhouse gas!
Comparing hydrogen fuels cells and H2 powered combustion engines, which would release more heat per kilogram of H2 combusted
Since H2 power combustion engines are less energy efficient, more heat energy is released (lost to the environment) per unit of H2 produced.
How is the hydrogen used in hydrogen fuel cells generally produced?
-From fossil fuels, in the process of steam reforming
What reacts in steam reforming?
Steam + fossil fuel ------> Carbon monoxide + Hydrogen gas, with Ni catalyst
steam reforming equation:
fossil fuel + H2O(g) ------> CO(g) + 3H2(g), Ni catalyst
CO(g) + H2O(g) -------> CO2(g) + H2(g), Cu or Fe as catalyst
Altnerative methods of hydrogen production:
-Using electrical energy to convert water to hydrogen (electrolysis), in a non-spontaneous redox reaction, where electrical energy is provided by a renewable source.
-Collecting biogas from landfill sites and converting the methane using steam reforming.
How is hydrogen commonly stored as?
Liquid or compressed gas
What is the boiling point of hydrogen gas and why is this problematic?
Hydrogen boils at -252oC so a lot of energy is used to liquefy it, making it very expensive to story as a liquid.
How is compressed hydrogen gas stored?
Stored in high-pressure tanks, which need to be larger than liquid hydrogen tanks to provide the same amount of energy.
Why isn't oxygen storage an issue?
Oxygen can be easily obtained from the surrounding air.
Advancements in hydrogen storage:
-There has been advancement in alternative methods of storing hydrogen generally called materials-based storage.
-These include
-Hydrogen adsorbing to the surface of materials such as metal hydrides
-Hydrogen can also dissociate and be absorbed into the lattice structure of some solid materials.
-Hydrogen can react reversibly with various chemicals.
Hydrogen safety considerations:
Hydrogen burns readily and a flame or spark will ignite it in air, causing an explosion. Hence, hydrogen must be stored in safe and robust containers, such as high-pressure tanks.
On the other hand, hydrogen has a low density so it rises rapidly and quickly disperes, often avoiding ignition and thus, explosions.
Function (P & S cells):
-To produce electical energy; they are particularly useful as a source of portable electrical energy.
Function (Fuel cells):
To produce electrical energy; they are particularly useful as a continuous source of high electric current.
Design features (Similarities):
-Both have a cathode, anode, electrolyte, electric current through the wire/conductor.
Design features Differences:
Reactants are stored in primary and secondary cells, whereas they are not stored in fuel cells and thus need a continuous supply from an external source.
-Fuel cells use fuels whereas reactants of galvanic cells can be electrodes or ions found in the electrolyte.
-Secondary cells can be recharged in a non-spontaneous redox reaction whereas fuel cells can't.
-In fuel cells, electrodes are separated but contains within the same vessel whereas in a galvanic cell, the electrodes are kept in separate vessels.
-Fuel cells don't have a salt bridge but have a more disperesed electrolyte, whereas galvanic cells have a designated salt bridge to house the electrolyte.
Energy transformation:
Both chemical to electrical
Do galvanic cells and fuel cells produce heat energy?
Yes, release some heat
Energy efficiency:
-Primary and secondary cells: 60-90%
-Fuel cells: 40-60%
Applications:
-Primary cells: energy for watches, remote controls, porable radios, cameras. Secondary cells: cars, mobile phones, cameras, computers.
-Fuel cells: cars, buses, commercial electricity generation, emergency back-up power supplies.
What is a lead-acid battery
Batteries used in cars that contains 6 cells connected in series.
-3 positive electrodes sandwiched between 4 negative electrodes.
Anode Conjugate Redox Pair:
Pb(s) / PbSO4(s)
Lead solid gets oxidised to lead sulfate.
Oxidation half equation:
Pb(s) + SO4 2- (aq) -----> PsSO4(s) + 2e-
Cathode Conjugate Redox Pair:
PbO2(s) / PbSO4(s)
Lead oxide gets reduced to lead sulfate
Reduction half equation:
PbO2(s) + SO4 2- (aq) + 4H+(aq) + 2e- ------> PbSO4(s) + 2H2O(l)