H Chem 1.1- Sections 1.2,1.3 and 1.6
5.0(1)
Card Sorting
1/38
Last updated 8:30 PM on 9/6/23
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
39 Terms
1
New cards
how is matter classified
physical state and composition
2
New cards
physical states of matter:
Solid, liquid, gas
3
New cards
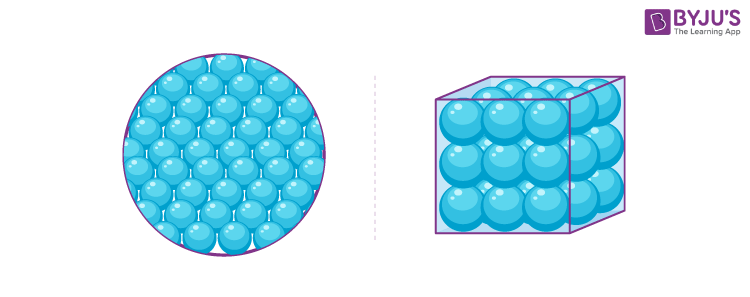
solid
has definite shape and volume
\-not compressible
\-atoms have a fixed position, and can only wiggle around.
\
\-not compressible
\-atoms have a fixed position, and can only wiggle around.
\
4
New cards
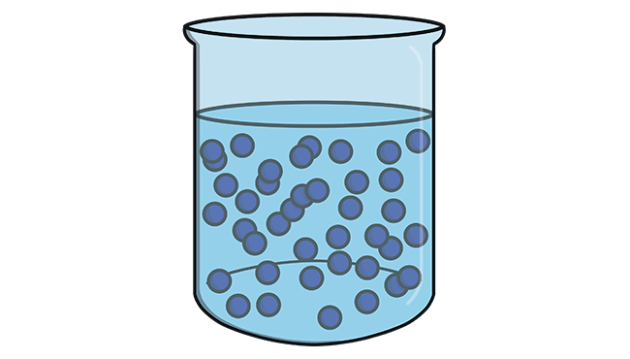
Liquid
no definite shape but definite volume
\-not compressible
\-atoms packed together and at a an equal distance to each other as a solid, but have no fixed position due to having enough energy to slide over each other, as seen as in pouring a liquid.
\
\-not compressible
\-atoms packed together and at a an equal distance to each other as a solid, but have no fixed position due to having enough energy to slide over each other, as seen as in pouring a liquid.
\
5
New cards
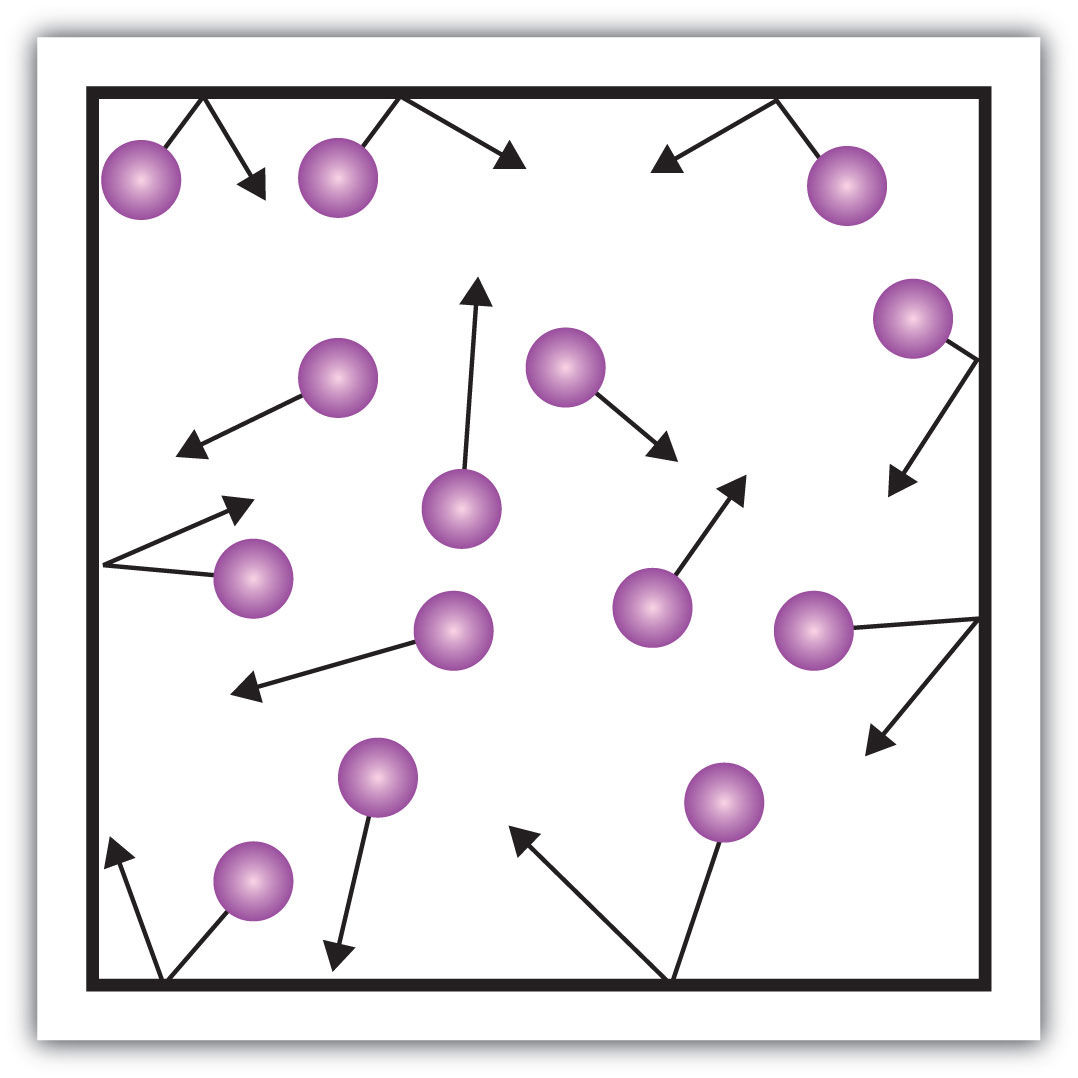
Gas
\-no definite shape or volume
\-compressible, so Amt of atoms stay the same when gas is put in a smaller or bigger container
\
\-compressible, so Amt of atoms stay the same when gas is put in a smaller or bigger container
\
6
New cards
what causes physical changes
thermal energy/temperature and pressure changes
7
New cards
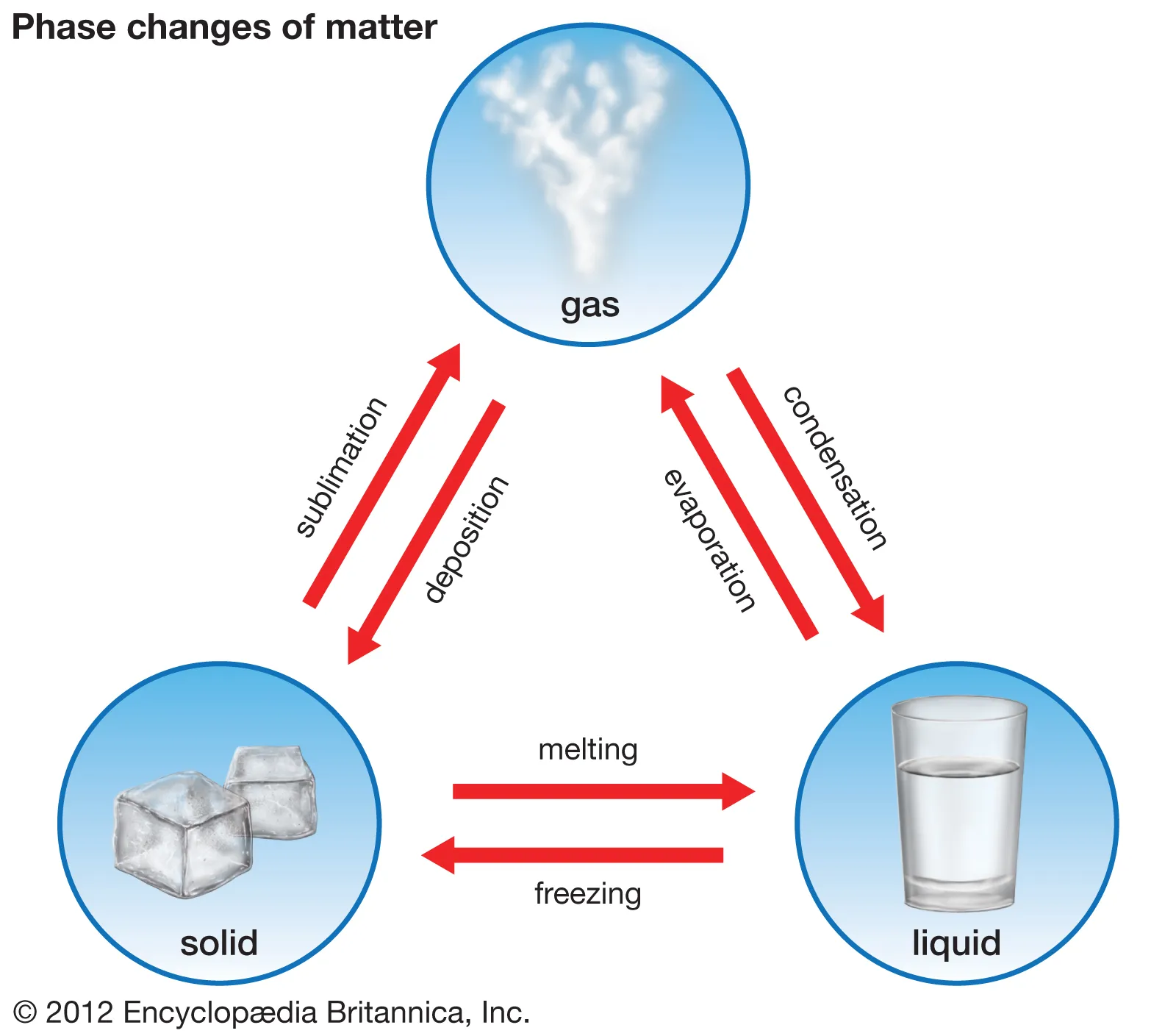
Names of physical state changes
Solid → gas: sublimation
Gas → solid: deposition
Solid → liquid: melting
liquid → solid: freezing
liquid → gas: evaporation
gas → liquid: condensation
Gas → solid: deposition
Solid → liquid: melting
liquid → solid: freezing
liquid → gas: evaporation
gas → liquid: condensation
8
New cards
types of composition
elements, compounds, mixtures
9
New cards
Elements
\-Cannot be broken down further
\-only __one__ kind of atom in substance
\-118 types, periodic table; some more present than others (74% H & 24% He in world; CHO = 90% of human)
\-some elements heavier than others due to amt. of protons or neutrons (or electrons)
\-only __one__ kind of atom in substance
\-118 types, periodic table; some more present than others (74% H & 24% He in world; CHO = 90% of human)
\-some elements heavier than others due to amt. of protons or neutrons (or electrons)
10
New cards
compounds
\-can be broken down into elements
\-2 or more atoms bonded intramolecularly together
\-elements interact with other elements interact to form compounds
Joe Louis proust’s Law of constant composition: no matter how a compound is made, its composition would be the same.
\-2 or more atoms bonded intramolecularly together
\-elements interact with other elements interact to form compounds
Joe Louis proust’s Law of constant composition: no matter how a compound is made, its composition would be the same.
11
New cards
molecule
group of atoms bonded together; can be either diatomic/triatomic/tetratomic atoms (2 or more of the same atoms bonded) or compounds (two or more different atoms bonded)
12
New cards
Pure substance
substance with only 1 type of molecule
\-either element or compound
\-no mixtures
\-either element or compound
\-no mixtures
13
New cards
mixtures
\-can be broken down into elements
\-2 or more different kinds of elements or compounds present together but not bonded to each other; each type of molecule retains its own identity.
\-can be bonded intermolecular, but not INTRAmolecularly
\-composition of mixture can vary
\-two types of mixtures
\-2 or more different kinds of elements or compounds present together but not bonded to each other; each type of molecule retains its own identity.
\-can be bonded intermolecular, but not INTRAmolecularly
\-composition of mixture can vary
\-two types of mixtures
14
New cards
types of mixtures
heterogenous and homogenous
15
New cards
heterogenous mixtures
\-visually distinguishable components; you can easily see how different the components are
ex. ice and water; orange juice with pulp; gold ore; sand; salad; oil and water
ex. ice and water; orange juice with pulp; gold ore; sand; salad; oil and water
16
New cards
homogenous mixtures
\-appear (But are not necessarily composed) uniformly throughout, meaning that the components in the mixture appear the same in the mixture; components are **not** visually distinguishable
ex. air; saltwater; paint; clay
ex. air; saltwater; paint; clay
17
New cards
properties of matter
how it reacts; what can be seen if/if not changed
\-physical properties and chemical properties
\-physical properties and chemical properties
18
New cards
physical properties
\-traits observed without changing a substance’s composition
\-ex. melting point, as applying thermal energy in a substance will not cause the substance to alter its composition.
\-ex. melting point, as applying thermal energy in a substance will not cause the substance to alter its composition.
19
New cards
chemical properties
\-things observed in how a substance can potentially change in composition
\-ex. oxidation; how easily oxygen can alter a substance into something else
\-ex. oxidation; how easily oxygen can alter a substance into something else
20
New cards
extensive and intensive properties of matter
chemical and physical properties can be extensive or intensive
21
New cards
intensive properties
when the chemical/physical property does not depend on the amt of sample
ex. color, density, melting point
melting point- if 1 in^2 of wax melts in 3 secs, and 4in^2 of wax melts in 12 secs; it is still melting at the same rate of 1in^2 every 3 secs
ex. color, density, melting point
melting point- if 1 in^2 of wax melts in 3 secs, and 4in^2 of wax melts in 12 secs; it is still melting at the same rate of 1in^2 every 3 secs
22
New cards
Extensive properties
\-physical/chemical property can only be seen/possible in a certain amount of substance
ex. weight; dimensions
dimensions- if 1kg of glass has an area of 1inch^2 and you’d want to see glass what is 5inch^2; you would only be able to do so with 5kg of glass; therefore the 5 inch^2 dimension in particular is an extensive property
ex. weight; dimensions
dimensions- if 1kg of glass has an area of 1inch^2 and you’d want to see glass what is 5inch^2; you would only be able to do so with 5kg of glass; therefore the 5 inch^2 dimension in particular is an extensive property
23
New cards
changes of matter
physical and chemical changes
24
New cards
physical change
\-a change in the appearance of matter, but not its composition.
\-ex melting wax will cause wax to become runny, but wax would still be wax;
shredding paper will cause the paper to become smaller in size and separate it, but it will still be paper
\-all physical states are due to physical changes
\-ex melting wax will cause wax to become runny, but wax would still be wax;
shredding paper will cause the paper to become smaller in size and separate it, but it will still be paper
\-all physical states are due to physical changes
25
New cards
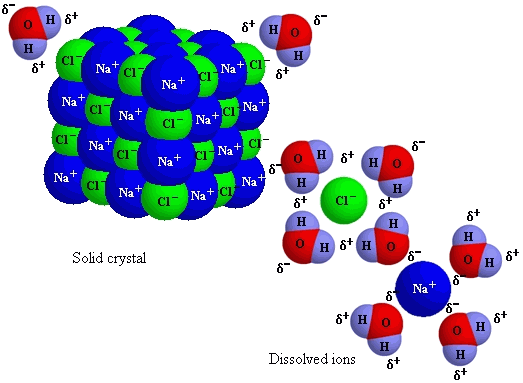
chemical changes
\-when the composition is changed in matter
ex. dissolving salt in water: water causes the bonded Na+Cl- ions to separate into un-bonded Na+ and Cl- ions
ex. dissolving salt in water: water causes the bonded Na+Cl- ions to separate into un-bonded Na+ and Cl- ions
26
New cards
separation of mixtures
\-mixtures are usually separated based on a differing property in the compositions within it.
ex. since the oil and water differ in boiling points; the Oil and water mixture can be separated through boiling the mixture until the water evaporated first and leaves just the oil behind
ex. since the oil and water differ in boiling points; the Oil and water mixture can be separated through boiling the mixture until the water evaporated first and leaves just the oil behind
27
New cards
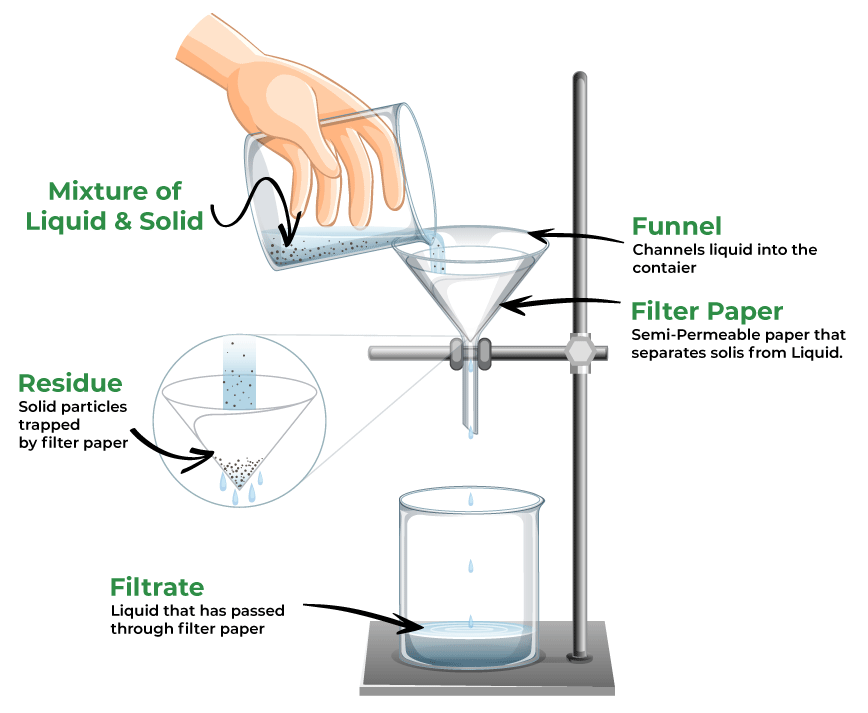
filtration
\-form of separating a mixture with components that are heterogenous or have diff physical states (liquid and solid)
\-the process of separating suspended solid matter from a liquid, by causing the liquid to pass through the pores of some substance, called a filter. the filter traps the solid and lets the liquid pass therefore separating them
\-mix is poured in funnel with filter paper; solid remains in the funnel as residue, liquid ends up in container and is known as the filtrate
\
\-the process of separating suspended solid matter from a liquid, by causing the liquid to pass through the pores of some substance, called a filter. the filter traps the solid and lets the liquid pass therefore separating them
\-mix is poured in funnel with filter paper; solid remains in the funnel as residue, liquid ends up in container and is known as the filtrate
\
28
New cards
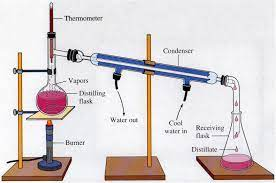
distillation
\-form of separating homogenous mixtures with components having diff boiling points (at least 25C difference)
boiling a mixture of components with different boiling points until the component(s) with the lower boiling points evaporate first and therefore leave behind the component(s) with the higher boiling point;
\--
component that evaporates first when mixture in distilling flask is heated is then condensed into another container, in Erlenmeyer flask; known as distillate → component with higher boiling point remains in distilling flask.
\-water is pumped in and out of condenser to lower temp of evaporated component into liquid state to be collected in Erlenmeyer flask.
\-parts of distillation: Distilling flask; condenser; Erlenmeyer flask; distillate.
\--
ex. since the oil and water differ in boiling points; the Oil and water mixture can be separated through boiling the mixture until the water evaporated first and leaves just the oil behind
\
boiling a mixture of components with different boiling points until the component(s) with the lower boiling points evaporate first and therefore leave behind the component(s) with the higher boiling point;
\--
component that evaporates first when mixture in distilling flask is heated is then condensed into another container, in Erlenmeyer flask; known as distillate → component with higher boiling point remains in distilling flask.
\-water is pumped in and out of condenser to lower temp of evaporated component into liquid state to be collected in Erlenmeyer flask.
\-parts of distillation: Distilling flask; condenser; Erlenmeyer flask; distillate.
\--
ex. since the oil and water differ in boiling points; the Oil and water mixture can be separated through boiling the mixture until the water evaporated first and leaves just the oil behind
\
29
New cards
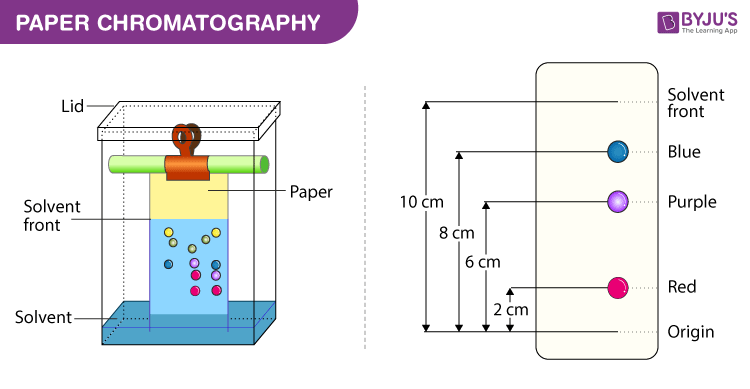
paper chromatography
\-separates homogeneous mixtures that differ in water or paper adherence
\-can also be used to identify unknown substances or solubility of substances.
\--
\-sample of mix is placed on chromatography paper/plate; a baseline (pencil mark) marks where the sample is in the beginning.
\-the chromatography paper (stationary phase) is then placed in a development chamber (beaker with lid) in a solvent; solvent climbs up to paper by capillary action mobile phase.
\-components in the sample mix will either dissolve or climb the paper in varying degrees depending on solubility or adherence of paper. Location where solvent is on chromatography paper/plate after a certain time determines solvent front.
\-higher up = more soluble and less adherent to paper as it easily follows the solvent’s flow
\-lower down = less soluble and more adherent to paper
\--
\-relationship of the distance moved by the component to distance moved by solvent → retention factor (Rf)
\-parts of chromatography: stationary phase, mobile phase, development chamber. baseline, solvent front; rf values.
\-can also be used to identify unknown substances or solubility of substances.
\--
\-sample of mix is placed on chromatography paper/plate; a baseline (pencil mark) marks where the sample is in the beginning.
\-the chromatography paper (stationary phase) is then placed in a development chamber (beaker with lid) in a solvent; solvent climbs up to paper by capillary action mobile phase.
\-components in the sample mix will either dissolve or climb the paper in varying degrees depending on solubility or adherence of paper. Location where solvent is on chromatography paper/plate after a certain time determines solvent front.
\-higher up = more soluble and less adherent to paper as it easily follows the solvent’s flow
\-lower down = less soluble and more adherent to paper
\--
\-relationship of the distance moved by the component to distance moved by solvent → retention factor (Rf)
\-parts of chromatography: stationary phase, mobile phase, development chamber. baseline, solvent front; rf values.
30
New cards
types of numbers in scientific work
exact and inexact numbers
31
New cards
exact numbers
\-defined values
ex. 100g= 1 kg; 2.2 lbs = 1kg; counting objects; 3 feet = yard
ex. 100g= 1 kg; 2.2 lbs = 1kg; counting objects; 3 feet = yard
32
New cards
inexact numbers
\-measuring → always inexact
can be due to human or equipment error every time (human setup or calibration will never be perfect/exact)
\-equipment error: equipment never show exact measurement as they display measurement to the nearest partial or full unit (nearest 1, 0.1, 0.00001 grams)
ex. machine that calculates to nearest 0.0001 grams, measurement is 2.6007, but it is not certain if real measurement was 2.60068, 2.60071, 2.600715686, etc.
\-human error: differences in how exactly humans measure every time
ex. human may have disposition ruler slightly to the left in measurement A and then slightly up in measurement B, or may have mis-calibrated or failed to calibrate machinery.
can be due to human or equipment error every time (human setup or calibration will never be perfect/exact)
\-equipment error: equipment never show exact measurement as they display measurement to the nearest partial or full unit (nearest 1, 0.1, 0.00001 grams)
ex. machine that calculates to nearest 0.0001 grams, measurement is 2.6007, but it is not certain if real measurement was 2.60068, 2.60071, 2.600715686, etc.
\-human error: differences in how exactly humans measure every time
ex. human may have disposition ruler slightly to the left in measurement A and then slightly up in measurement B, or may have mis-calibrated or failed to calibrate machinery.
33
New cards
used to discuss uncertainness of measured values
precision and accuracy
34
New cards
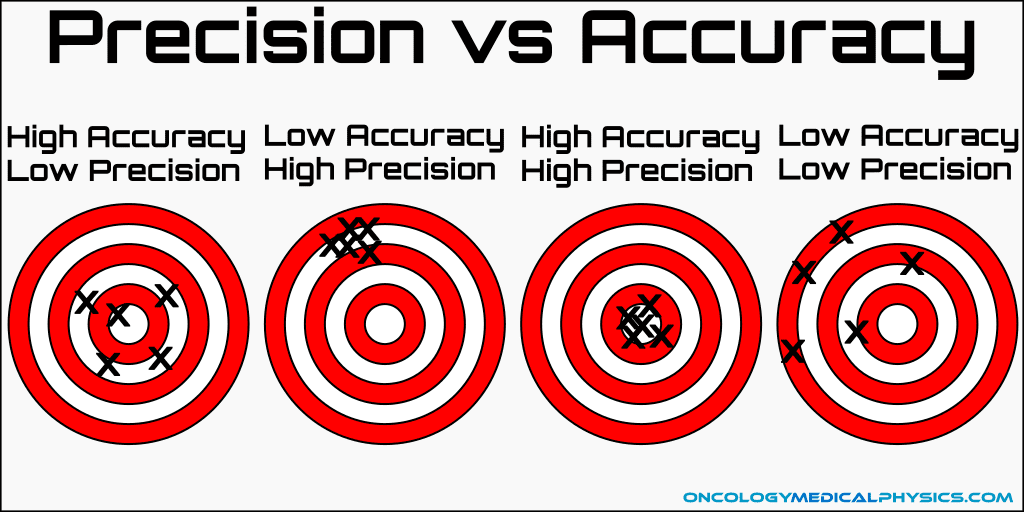
precision:
\-how closely do a set of measurements of a certain item agree with each other
ex. measurements of 1 kg in a 1in. cube of ice,2kg in 2in. , and 5kg in 5in. of ice show that these measurements are precise, as the amount of mass changing to the size of the ice in each measurement agree with each other.
\--
precision DOES NOT mean accuracy; if precise but inaccurate then that could show equipment or human error in measuring
ex. measurements of 1 kg in a 1in. cube of ice,2kg in 2in. , and 5kg in 5in. of ice show that these measurements are precise, but true measurement of ice cubes should 0.5 kg in 1in, 1 kg in 2in, and 2.5 kg in 5in show that these measurements are inaccurate.
ex. measurements of 1 kg in a 1in. cube of ice,2kg in 2in. , and 5kg in 5in. of ice show that these measurements are precise, as the amount of mass changing to the size of the ice in each measurement agree with each other.
\--
precision DOES NOT mean accuracy; if precise but inaccurate then that could show equipment or human error in measuring
ex. measurements of 1 kg in a 1in. cube of ice,2kg in 2in. , and 5kg in 5in. of ice show that these measurements are precise, but true measurement of ice cubes should 0.5 kg in 1in, 1 kg in 2in, and 2.5 kg in 5in show that these measurements are inaccurate.
35
New cards
standard deviation
how much 1 certain measurement differs from average in set of measurements
\-small deviation = more precise and valid
\-small deviation = more precise and valid
36
New cards
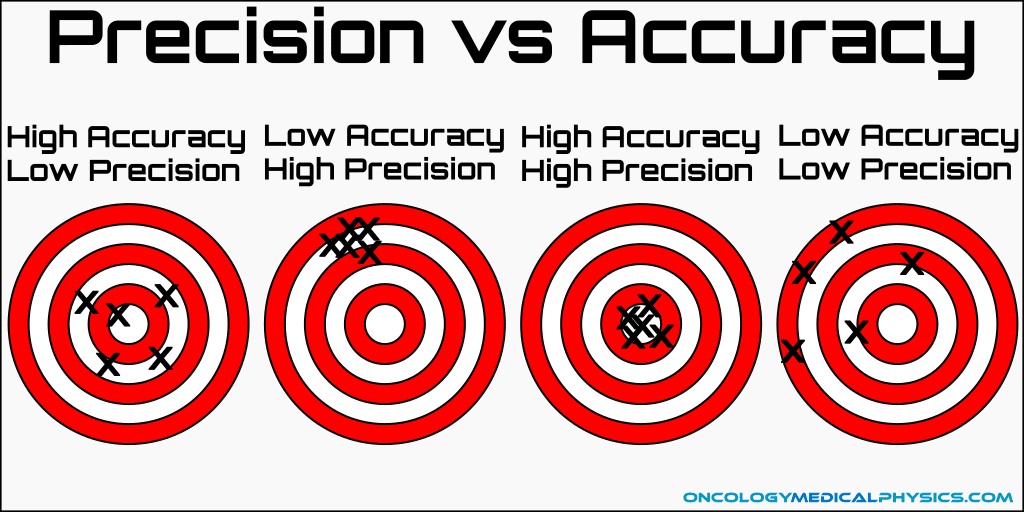
accuracy
how closely measurements agree with true measurement
ex. measurement is not accurate because you measure that 1in of ice is 1kg and the true given measurement is 0.5 kg in 1 inch.
\--
accuracy DOES NOT equal precision; can indicate human/equipment error in one or more of the measurements
ex. measurement is not accurate because you measure that 1in of ice is 1kg and the true given measurement is 0.5 kg in 1 inch.
\--
accuracy DOES NOT equal precision; can indicate human/equipment error in one or more of the measurements
37
New cards
significant values
when measuring, all values are uncertain
ex. machine that calculates to nearest 0.0001 grams, measurement is 2.6007, but it is not certain if real measurement was 2.60068, 2.60071, 2.600715686, etc., and if the machine rounded the last digit up or down, so you would write it as 2.6007 +/- 0.0001
\-since it is agreed that all values are uncertain, measurements are usually reported without the +/- as it is assumed that it is uncertain.
\
\-any measured value with digits
ex. machine that calculates to nearest 0.0001 grams, measurement is 2.6007, but it is not certain if real measurement was 2.60068, 2.60071, 2.600715686, etc., and if the machine rounded the last digit up or down, so you would write it as 2.6007 +/- 0.0001
\-since it is agreed that all values are uncertain, measurements are usually reported without the +/- as it is assumed that it is uncertain.
\
\-any measured value with digits
38
New cards
how to determine significant values
\-any nonzero digit is significant (ex 1999 has 4 sig figs)
\-any zeros in between nonzero digits are significant (2008 has 4 sig figs)
\-any zeros that are before nonzero digits are NOT significant (0.08 has 1 sig fig)
\-any zeros after a decimal number are significant (900.0 has 4 sig figs)
\-any zeros after a non decimal number are NOT sig figs (900 has 1 sig fig)
\-any number known as being EXACT, such as counting the amt of something, will have INFINITE significant figures
\-any zeros in between nonzero digits are significant (2008 has 4 sig figs)
\-any zeros that are before nonzero digits are NOT significant (0.08 has 1 sig fig)
\-any zeros after a decimal number are significant (900.0 has 4 sig figs)
\-any zeros after a non decimal number are NOT sig figs (900 has 1 sig fig)
\-any number known as being EXACT, such as counting the amt of something, will have INFINITE significant figures
39
New cards
mixtures can be distillates only if the boiling point differs by:
25 degrees C