Module 10: Carboxylic Acid Derivatives
1/32
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
33 Terms
To name esters you find the longest continuous chain attached to the _____ and add the suffix ___. This is the parent chain
carbonyl group, -oate
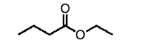
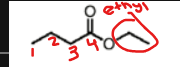
name this molecule
ethyl butanoate
to name amides, you find the longest continuous chain attached to the ____ and add the ending____. This is the parent chain. At the beginning of the name, you need to add an ___ to designate which atom the substituent is attached to.
carbonyl group, amide, N
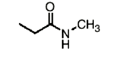
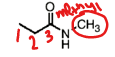
Name this molecule
N-methyl propanamide
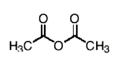
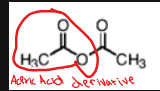
Name this molecule
acetic anhydride
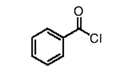
name this molecule
benzoyl chloride
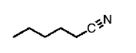
name this molecule. To name nitriles, count the longest continuous chain starting from the carbon attached to the nitrile. Youd do NOT drop the -e in this case
hexanenitrile
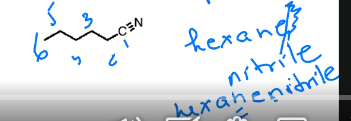
the carbonyl group will be more reactive when it is not ____.
stabilized
The more positive the carbonyl group, the more -___ it is.
reactive
rank amides, anhydrides, esters, and acyl halides from most stable to lest stable
amides > esters > anhydrides > acyl halides
rank amides, anhydrides, esters, and acyl halides from most reactive to least reactive.
acyl halides > anhydrides > esters > amides
the more stable an acyl carbon is, the ___ reactive the molecule will be.
less
the less stable an acyl carbon is, the ___ reactive the molecule will be.
more
one carboxylic acid derivative can be converted to another if the reaction leads to a more ____ carbonyl group.
stabilized
you can convert acyl chlorides into ?
anhydrides, esters, and amides
you can convert anhydrides into?
esters and amides
esters can be converted into?
amides
esters cannot be converted into ?
anhydrides or acyl halides, they are less stable than esters
anhydrides cannot be converted into?
acyl halides, they are less stable than anhydrides
amides cannot be converted into?
anhydrides, esters, or acyl halides, they are less stable than amides
draw the structure for acetic anhydride

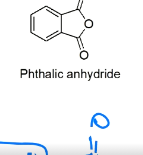
draw the structure for Phthalic anhydride
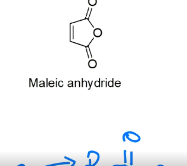
draw the structure for Maleic anhydride
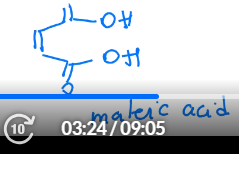
draw the structure for maleic acid
cyclic anhydrides are the only way to close __ or __ member rings
5 or 6
for the hydrolysis of esters in acidic conditions, each step in the mechanism is ____.
reversible
for the hydrolysis of esters in basic conditions, all steps are irreversible exceot for ___.
the last step
ester reactions with amines to get amides do not work with ____ amines.
tertiary
increasing the alkyl chain on the oxygen of an ester ____ the reactivity of that side of the molecule
decreases.
you can react esters with __, ___, and ___ to get amides.
ammonia, primary amines, and secondary amines
Lactams are _____.
cyclic amides
cyclic amides are called?
lactams
amides are not very ____.
reactive