3.6. CARBOXYLIC ACIDS AND DERIVATIVES
1/14
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
15 Terms
How is benzoic acid produced from an alkylbenzene such as methylbenzene?
Oxidation of Alkylbenzenes
Reagents: Hot alkaline KMnO₄ followed by dilute acid (HCl).
Conditions: Reflux.
Observation: The purple Mn⁷⁺ ions are reduced to Mn⁴⁺, forming a brown MnO₂ precipitate.
Final Step: Acidification with dilute HCl protonates the organic product → Benzoic acid (C₆H₅COOH) is formed.
Equation: C₆H₅CH₃ + [O] → C₆H₅COOH
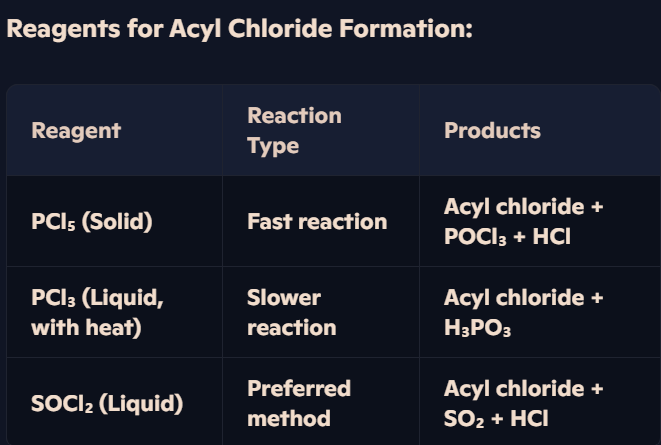
How do carboxylic acids react with PCl₃, PCl₅, or SOCl₂ to form acyl chlorides?
Formation of Acyl Chlorides (-COCl)
Carboxylic acids react with:
Solid PCl₅ → Acyl chloride + POCl₃ + HCl.
Liquid PCl₃ + Heat → Acyl chloride + H₃PO₃.
Liquid SOCl₂ → Acyl chloride + SO₂ + HCl.
Example – Formation of Ethanoyl Chloride:
CH₃COOH + PCl₅ → CH₃COCl + POCl₃ + HCl
How is methanoic acid (HCOOH) further oxidised, and what observations can be made?
Oxidation of Methanoic Acid (HCOOH):
Methanoic acid acts as a reducing agent and undergoes oxidation to CO₂.
Oxidising Agents Used:
Fehling’s solution: Cu²⁺ reduced to Cu₂O (red precipitate).
Tollens’ reagent: Ag⁺ reduced to metallic silver (mirror effect).
Acidified KMnO₄ or K₂Cr₂O₇: Mn⁷⁺ → Mn²⁺ (Purple → Colourless) / Cr⁶⁺ → Cr³⁺ (Orange → Green).
Equation: HCOOH + [O] → CO₂ + H₂O
How is ethanedioic acid (HOOCCOOH) oxidised, and what is the role of KMnO₄?
Oxidation of Ethanedioic Acid:
Reagent: Warm acidified KMnO₄.
Products: Carbon dioxide (CO₂) & water.
Observations: Mn⁷⁺ ions are reduced to Mn²⁺, causing the purple KMnO₄ solution to turn colourless.
Equation: HOOCCOOH + [O] → 2CO₂ + H₂O
How do the acidities of carboxylic acids, phenols, and alcohols compare, and what factors influence their relative strengths?
Factors Affecting Acidity:
Strength of O-H Bond:
In carboxylic acids, the carbonyl (-C=O) group withdraws electron density, weakening the O-H bond.
Alcohols lack this electron-withdrawing effect, making them weaker acids.
Stability of Conjugate Base:
Carboxylate ions (-COO⁻) are highly stable due to charge delocalisation, making carboxylic acids stronger acids.
Phenoxide ions (C₆H₅O⁻) are stabilised but not as effectively as carboxylates → Weaker acidity than carboxylic acids.
Alkoxide ions (C₂H₅O⁻) are destabilised due to electron donation from the alkyl group → Ethanol is the weakest acid.
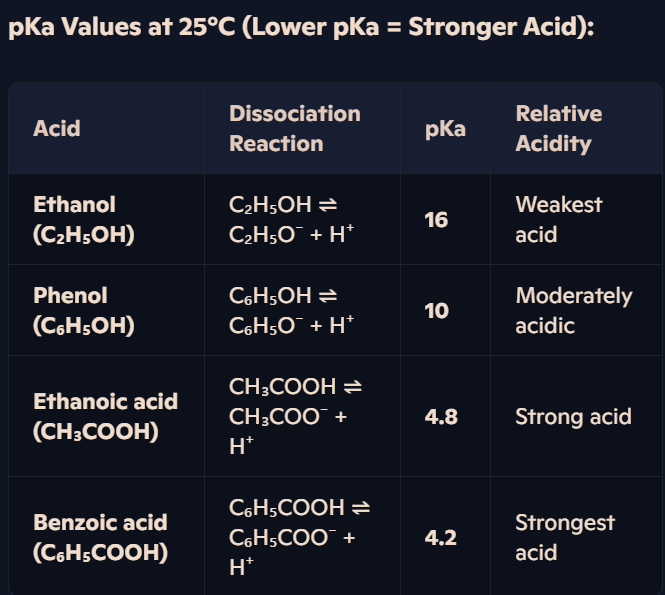
How do electron-withdrawing chlorine atoms affect the acidity of carboxylic acids?
Why More Chlorine = Stronger Acidity?
Chlorine atoms withdraw electron density from the O-H bond, making it weaker and facilitating proton loss.
Delocalisation of charge in the carboxylate ion is further extended by Cl atoms → Greater stability → Lower tendency to recombine with H⁺.
Example – Trichloroethanoic Acid (Strongest Acid):
Three electronegative Cl atoms withdraw electron density.
Highly stabilised carboxylate ion (-COO⁻) reduces attraction to H⁺ → Very strong acid.
What are esters, and why are acyl chloride reactions preferred for their synthesis?
Structure & Uses of Esters:
Esters have the -COOR functional group.
Used in perfumes, cosmetics, and solvents due to their characteristic smells.
Named based on their reactants:
Alcohol contributes the first part of the name.
Acyl chloride contributes the second part (e.g., Ethyl ethanoate = ethanol + ethanoyl chloride).
Why Use Acyl Chlorides Instead of Carboxylic Acids?
Acyl chlorides are more reactive → Faster ester formation.
Reaction goes to completion → No equilibrium mixture, maximum ester yield.
How does ethanol react with ethanoyl chloride to form ethyl ethanoate?
Reaction Details:
Reagents: Ethanol (C₂H₅OH) + Ethanoyl chloride (CH₃COCl).
Products: Ethyl ethanoate (CH₃COOCH₂CH₃) + HCl (white fumes).
Mechanism:
Nucleophilic attack by ethanol’s -OH group on the electron-deficient carbonyl carbon.
Chlorine (-Cl) atom is eliminated, forming HCl gas.
Ester bond (-COO-) is formed, producing ethyl ethanoate.
Equation: CH₃COCl + C₂H₅OH → CH₃COOCH₂CH₃ + HCl
How does phenol react with benzoyl chloride to form phenyl benzoate?
Reaction Details:
Reagents: Phenol (C₆H₅OH) + Benzoyl chloride (C₆H₅COCl).
Products: Phenyl benzoate (C₆H₅COOC₆H₅) + HCl.
Mechanism:
Phenol’s -OH group attacks the carbonyl carbon in benzoyl chloride.
Chlorine (-Cl) is eliminated, releasing HCl gas.
Phenyl benzoate (ester) is formed.
Equation: C₆H₅COCl + C₆H₅OH → C₆H₅COOC₆H₅ + HCl
How are acyl chlorides synthesized from carboxylic acids, and what reagents are required?
Acyl Chlorides & Their Reactivity:
Acyl chlorides contain the -COCl functional group.
They are more reactive than carboxylic acids, making them valuable intermediates in organic synthesis.
Example – Formation of Propanoyl Chloride:
CH₃CH₂COOH + SOCl₂ → CH₃CH₂COCl + SO₂ + HCl
How do acyl chlorides react with water, and what is the mechanism?
Reaction Overview:
Reagent: H₂O (room temperature).
Products: Carboxylic acid + HCl gas.
Observations: White fumes of HCl are released.
Mechanism:
Water’s lone pair attacks the carbonyl carbon.
Addition of H₂O molecule.
Elimination of HCl, forming the carboxylic acid.
Equation: CH₃CH₂COCl + H₂O → CH₃CH₂COOH + HCl
How do acyl chlorides react with alcohols and phenols to form esters?
Reaction with Alcohols:
Reagent: Alcohol (room temperature).
Products: Ester + HCl.
Example: Ethanol + Ethanoyl chloride → Ethyl ethanoate + HCl.
Equation: CH₃COCl + C₂H₅OH → CH₃COOCH₂CH₃ + HCl
Reaction with Phenols:
Reagent: Phenol + Base (NaOH, needed for phenoxide formation).
Products: Ester + NaCl.
Example: Phenol + Benzoyl chloride → Phenyl benzoate + NaCl.
Equation: C₆H₅COCl + C₆H₅O⁻ → C₆H₅COOC₆H₅ + NaCl
How do acyl chlorides react with ammonia and amines to form amides?
Reaction with Ammonia (NH₃):
Reagents: NH₃ (room temperature).
Products: Non-substituted amide + HCl gas.
Example: Ethanoyl chloride + NH₃ → Ethanamide + HCl.
Equation: CH₃COCl + NH₃ → CH₃CONH₂ + HCl
Reaction with Primary & Secondary Amines:
Reagent: Amine (room temperature).
Products: Substituted amide + Ammonium salt.
Example: Ethanoyl chloride + Methylamine → N-Methylethanamide + Methylammonium chloride.
Equation: CH₃COCl + CH₃NH₂ → CH₃CONHCH₃ + CH₃NH₃⁺Cl⁻
What is the general addition-elimination mechanism for acyl chloride reactions?
Step 1 – Addition of a Nucleophile:
Water, alcohol, phenol, ammonia, or amine attacks carbonyl carbon using a lone pair.
Electron transfer weakens C=O bond.
Step 2 – Elimination of a Small Molecule:
Leaving group (-Cl) is expelled, forming HCl gas or NaCl (with phenols).
Final Product: Carboxylic acid, ester, or amide.
Example – Hydrolysis of Acyl Chlorides:
Nucleophilic attack by water.
Formation of intermediate.
Elimination of HCl, forming carboxylic acid.