Module 11 Online HW Review Bonus
1/29
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
30 Terms
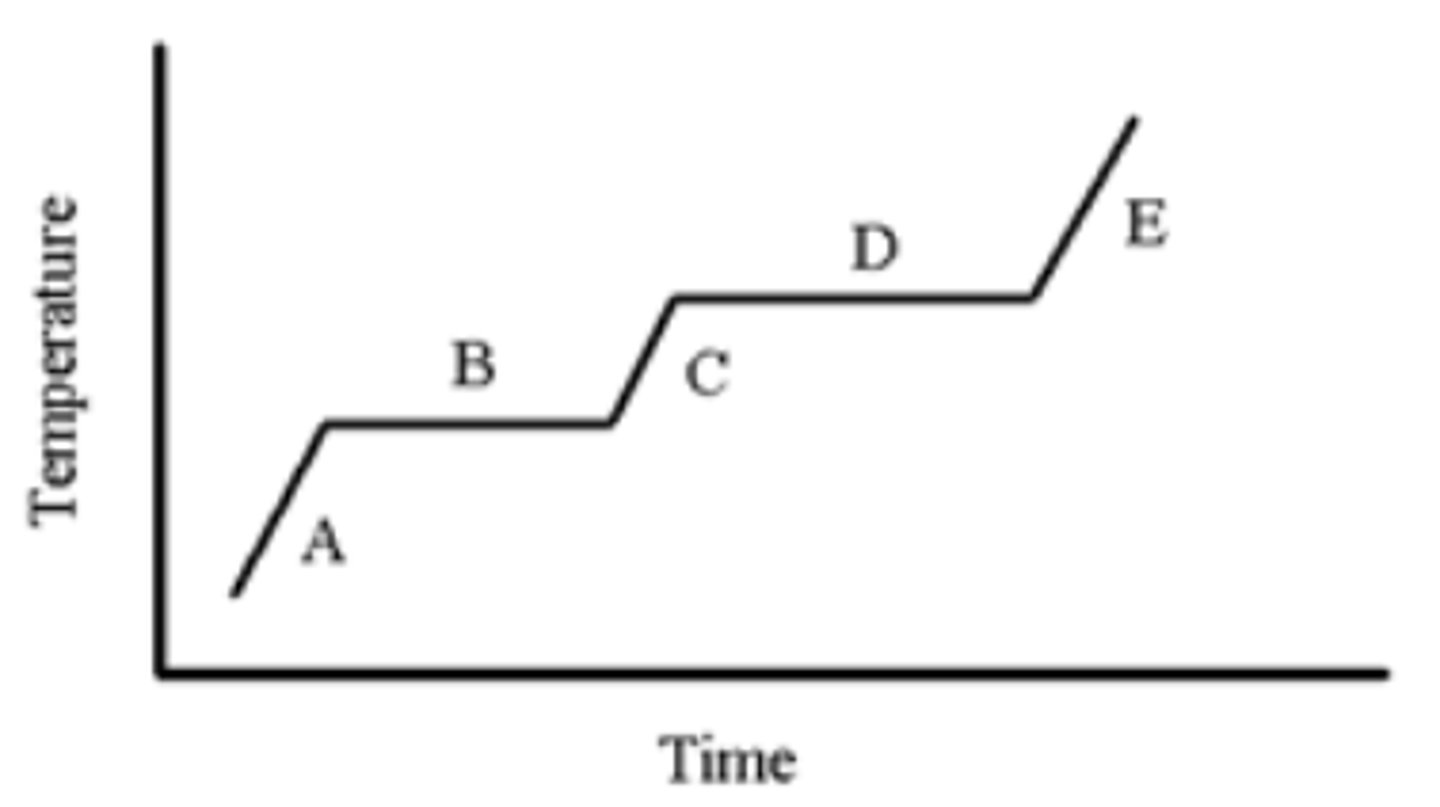
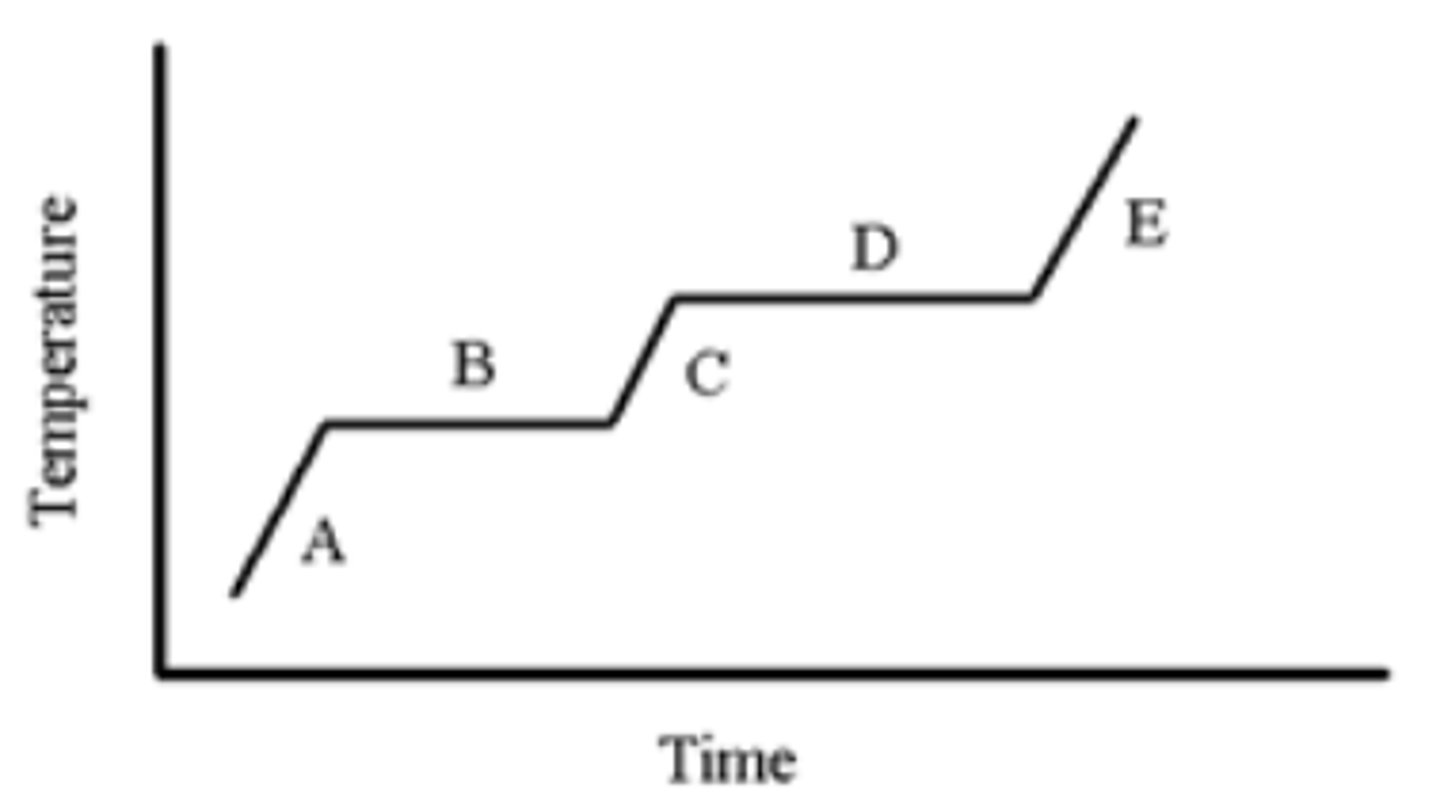
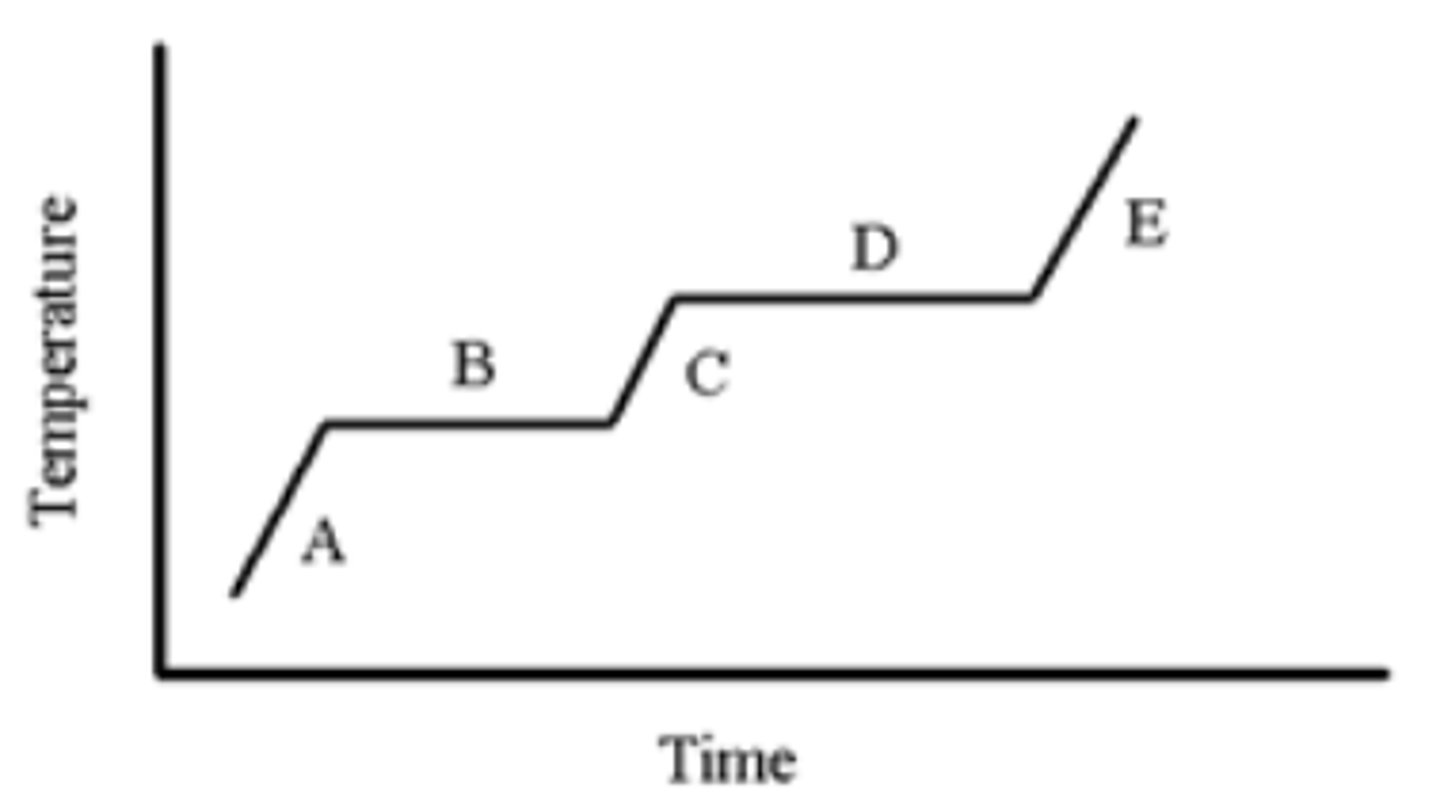
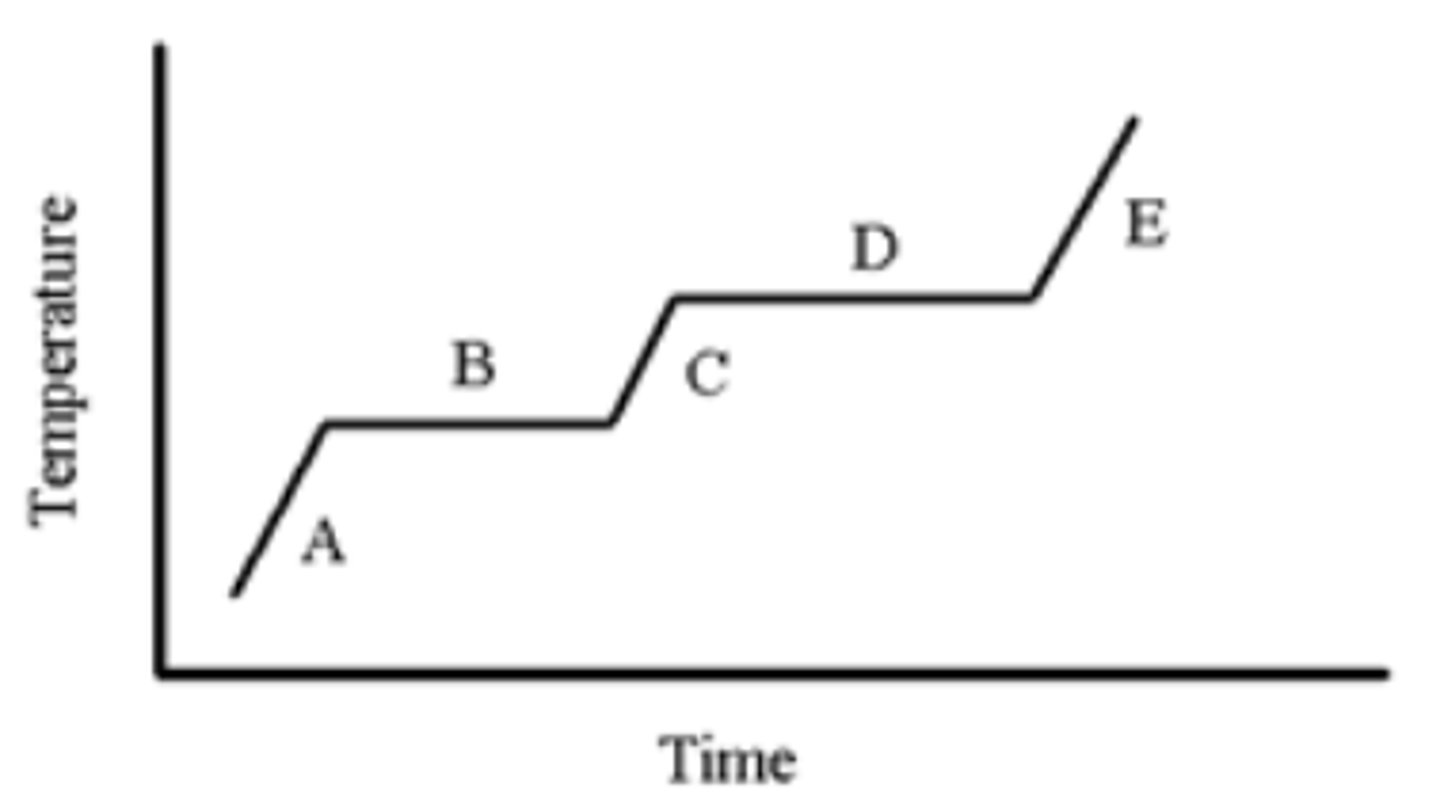
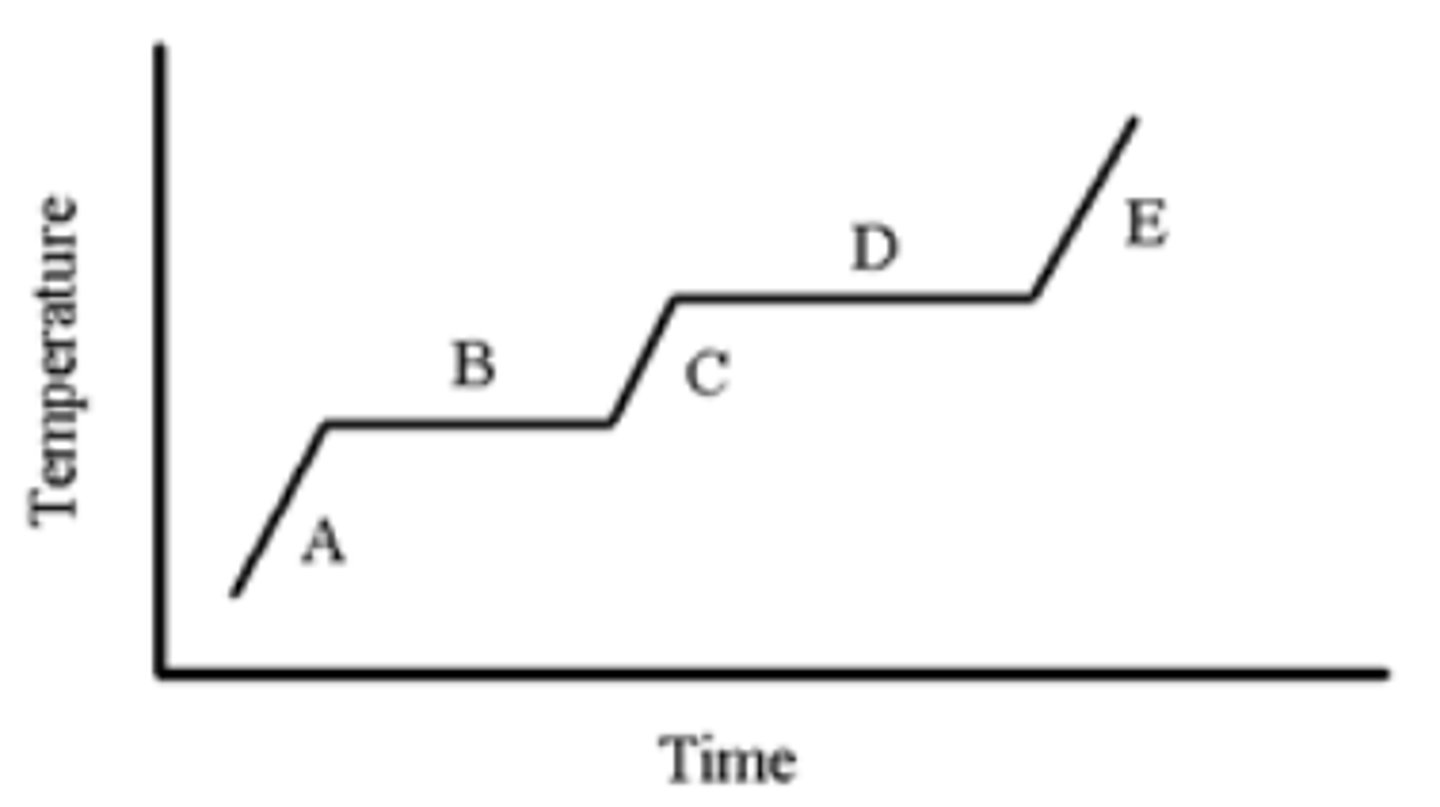
A pure substance is heated as indicated in the heating-cooling curve below. On which portion(s) of the graph is a solid present?
A,B
3 multiple choice options
A student performed an experiment to determine the ratio of H2O to CuSO4 in a sample of hydrated copper(II) sulfate by heating it to drive off the water and weighing the solid before and after heating. The formula obtained experimentally was CuSO4•5.5H2O but the accepted formula is CuSO4•5H2O. Which error best accounts for the difference in results?
During heating some of the hydrated copper(II) sulfate was lost.
3 multiple choice options
A pure substance is heated as indicated in the heating-cooling curve below. Which portion of the graph indicates the freezing point?
B
3 multiple choice options
If you have 300. grams of water at 100oC. How many kilojoules of heat are required to evaporate completely all the water? ΔHvap of water is 40.7 kJ/mole.
678 kJ
3 multiple choice options
Which of the following is not a generally observed property of solids?
less dense than liquids
3 multiple choice options
In liquid methanol, CH3OH, which intermolecular forces are present?
Dispersion, hydrogen bonding, and dipole-dipole forces are present.
3 multiple choice options
The following list of properties is most descriptive of a(n) ______________. High melting point, conductor of electricity when dissolved in water.
ionic solid
3 multiple choice options
If you wanted to synthesize a compound that had a fairly low melting point and a low density, you would want to synthesize:
a molecular solid.
1 multiple choice option
Which liquid has the lowest boiling point?
CH3OCH3
3 multiple choice options
O2 and O3 are ________ of oxygen.
allotropes
3 multiple choice options
When 0.400 mole of potassium reacts with excess water at standard temperature and pressure as shown in the equation above, the volume of hydrogen gas produced is:
2 K (s) + 2 H2O (l) → 2 K+ (aq) + 2 OH- (aq) + H2 (g)
4.48 L
3 multiple choice options
A pure substance is heated as indicated in the heating-cooling curve below. On which portion of the graph is only a liquid present?
C
3 multiple choice options
Which of the following statements is true?
Molecular solids have a low density.
3 multiple choice options
The name for CuSO4·5H2O is:
copper(II) sulfate pentahydrate.
3 multiple choice options
The following list of properties is most descriptive of a(n) ______________. Malleable, ductile, insoluble in water.
metallic solid
3 multiple choice options
The value of ΔoHfus for bromine is 10.6 kJ/mol. To which process does this value refer?
Br2 (s) → Br2 (l)
3 multiple choice options
When a hydrate of Na2CO3 is heated until all the water is removed, it loses 54.3% of its mass. The formula of the hydrate is:
Na2CO3•7H2O
3 multiple choice options
Which of the following is not an observed property of liquids?
compress significantly
3 multiple choice options
What is the percent water in hydrated calcium chloride (CaCl2•2H2O)?
24.51%
3 multiple choice options
Which is expected to have the largest dispersion forces?
C8H18
3 multiple choice options
The following list of properties is most descriptive of a(n) ______________. Low melting point, non-conductor of electric current.
molecular solid
3 multiple choice options
Consider the following list of liquids and their boiling points:
ether, bp 35°C acetone, bp 56°C cyclohexane, bp 80°C water, bp 100°C
Which liquid is predicted to have the largest vapor pressure near room temperature (25° C)?
ether
3 multiple choice options
A pure substance is heated as indicated in the heating-cooling curve below. On which portion(s) of the graph would you use the formula, (Csubstance)(m)(ΔT), to calculate the energy change?
A,C,E
3 multiple choice options
When a liquid is heated at its boiling point, the:
temperature of the liquid remains the same as long as any liquid is present.
3 multiple choice options
If 2.68 g of hydrated sodium sulfate, Na2SO4•nH2O, on heating produces 1.26 g of water, what is the empirical formula of this compound?
Na2SO4•7H2O
3 multiple choice options
Which of the following statements concerning ionic compounds is true?
Essentially all ionic compounds are solids at room temperature and pressure.
3 multiple choice options
A pure substance is heated as indicated in the heating-cooling curve below. On which portion of the graph is only a gas present?
E
3 multiple choice options
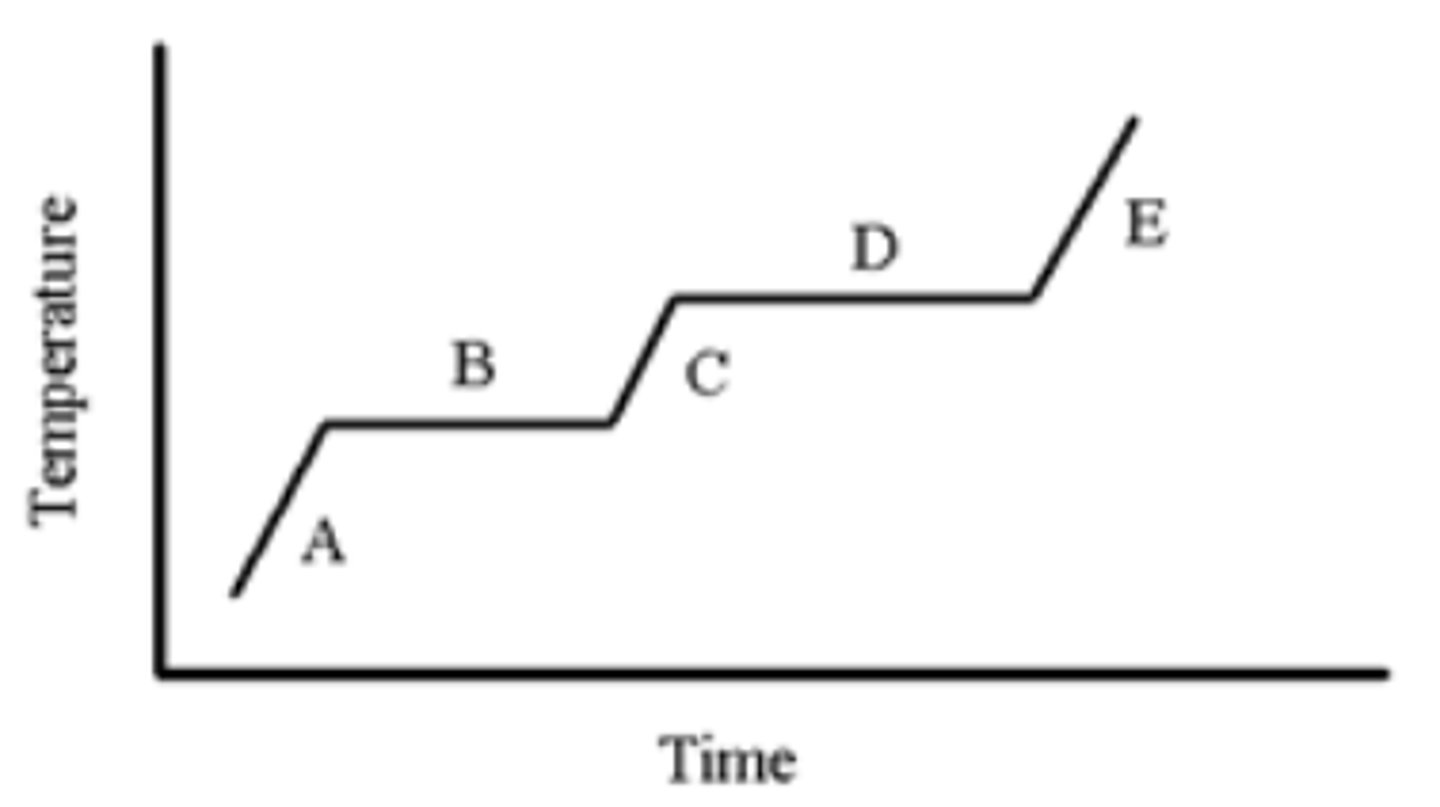
A pure substance is heated as indicated in the heating-cooling curve below. Which portion of the graph indicates the melting point?
B
3 multiple choice options
Which liquid has the highest boiling point?
CH3COOH
3 multiple choice options
Ethyl chloride, C2H5Cl, is used as a local anesthetic. It works by cooling tissue as it vaporizes. The heat of vaporization is 26.4 kJ/mol. How much heat could be removed by 10.0 g of ethyl chloride?
4.09 kJ
3 multiple choice options