stereochemistry
1/12
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
13 Terms
H/H eclipsed energy cost:
Torsional strain: 4 KJ/mol
H/CH3 eclipsed energy cost:
Torsional strain: 6 KJ/mol
H3C/CH3 eclipsed energy cost:
Torsional strain + steric interaction: 11 Kj/mol
CH3/CH3 exlipsed energy cost:
Steric interaction: 3.8 KJ/mol
The Baeyer strain theory
it was thought that rings were flat. It states that larger rings would be very highly
strained, as their bond angles would be very different from the
optimum 109.5°
It turns out that cycloalkanes with more than three C atoms in the
ring are not flat molecules. They are:
puckered to reduce strain
what is the most stable cycloalkane?
cyclohexane
heat of combustion vs stability
The less heat released, the more stable
axial and equatorial rings
axial is up down, equatorial is left right
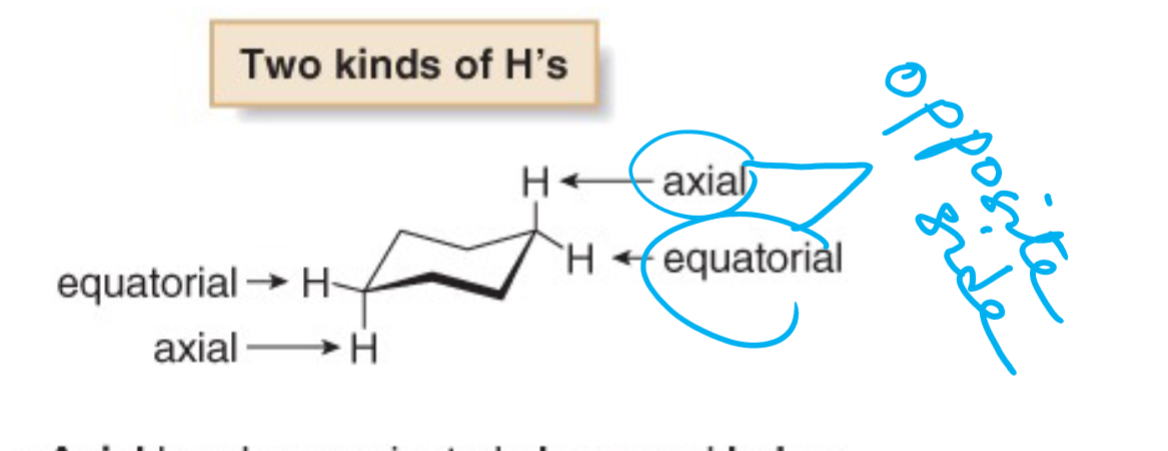
Which is more stable- axial or equatorial?
equatorial bc bulky groups on it cause less strain
diastereomers
isomers that aren’t mirror images. ex. cis and trans are diastereomers
number of stereoisomers
2^n (n=# of assymetric centers)