Electrode potentials + Electrochemical series + Cell notation
1/15
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
16 Terms
What does a more positive E0 value tell you about the species?
It means it is more likely to be reduced
What does a more negative E0 value tell you about the species?
It means it is more likely to be oxidised
What happens to reducing agents in the context of redox reactions?
They get oxidised
What does the reducing agent do to the other species?
Reduces the other species
Which hand of the equation will the reducing agent be found on in the electrochemical series?
Right hand
What is the trend for reducing agents (in terms of reducing power)?
The more negative the E0 value, the stronger the reducing power
What happens to the oxidising agent in the context of redox reactions?
Gets reduced
What does the oxidising agent do to the other species?
It oxidises the other species.
Which hand of the equation will the oxidisng agent be found on in the electrochemical series?
Left hand
What is the trend for oxidising agents (in terms of oxidising power)?
The more positive the E0 value, the stronger the oxidising power
What does a positive E0 cell value tell you about the feasibility of a reaction?
It tells you that the reaction is feasible.
If E0 cell is positive, what does this mean for ΔS and lnk?
Will be positive!
How does equilibrium position affect value of E0? Mention what will happen to the E0 value when equilibrium position is shifted to the right and shifted to the left

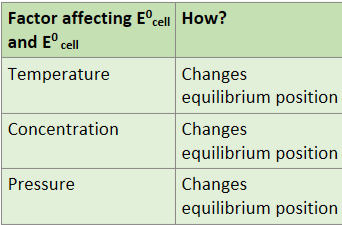
What are factors that affect E0 cell and E0 values?
What are reasons for a reaction not being feasible even when E0cell is positive?
Non-standard conditions
High activation energy
Rate of reaction very slow
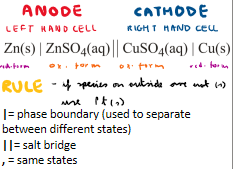
What are the rules for cell notation? Which electrode is on which side of the equation?