Chapter 4: Nutrient Metabolism
1/21
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
22 Terms
Liver
Receives
blood from intestinal tract (monosacharides and AA)
hormones from pancreas (insulin and glucagon)
Brain
blood-brain barrier: prevents acces of lipid-soluble (hydrophobic) molecules to the brain, for example non-esterified FA
relies on glucose or ketone bodies (in starvation)
Muscle tissue
= buffer store of phosphocreatine (= creatine phosphate)
in equilibrium with ATP through action of creatine kinase
Both contain protein
oxidative/ red fibres | white fibres |
|
|
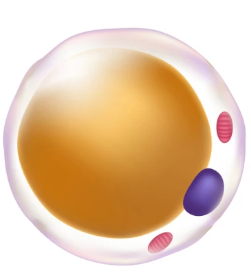
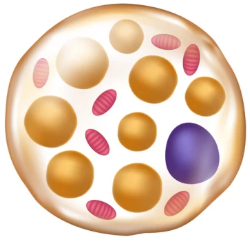
Fat tissue
White adipose tissue | Brown adipose tissue | |
mitrochondrial density | Low | High |
Lipid droplets | Single, large | Multiple, small |
Primary function | Energy storage (triglycerides → FA) | Thermogenesis (= heat production) by uncoupling fat oxidation from ATP production |
 |  |
kidneys
renal arteries: supply blood to kidneys
renal veins: return blood from kidneys
cortex | medulla | |
blood supply | high | low |
metabolism | aerobic metabolism (oxidation of glucose, FA and ketone bodies) | anaerobic metabolism of glucose |
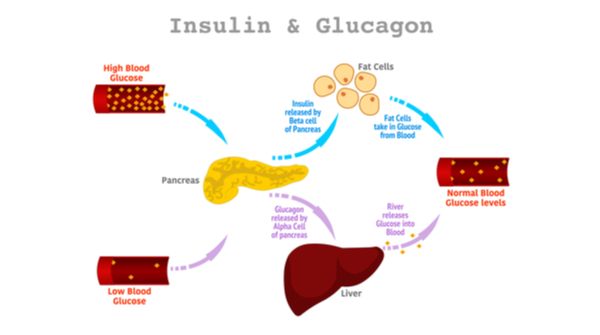
Regulation of energy metabolism (plasma glucose level maintenance)
1) Pancreas: islands of Langerhans
function: Maintenance plasma glucose at constant level
insulin | glucagon | |
location of production | beta-cells | alpha-cells |
stimulans |
|
|
Surpressor | / |
|
Reaction |
|
|
2) Adrenal medulla
function: secretes catecholamines
Adrenaline / epinephrine
Noradrenaline/ norepinephrine
Increase activity of
Glycogen phosphorylase: glycogen → glucose (+: glucagon, -: insulin)
Hormone sensitive lipase: lipase in adipocytes which liberates FA from TAG (+: glucagon, -: insulin)
Carbohydrate metabolism
Steps before
1) Digestion of dietary carbohydrates (starch, sucrose, lactose) —> glucose, fructose, galactose
2) Absorbed and transported to the liver by the hepatic portal vein
3)
part of fructose → glucose (intestinal epithelial cells)
galactose + remaining fructose → glucose (liver)
Characteristics
blood glucose concentration = dynamic equilibrium = around 5 mmol/L
dysfunction below 3 or above 11 mmol/L
No G6P in muscle
Glucose synthesis from fat not possible
Limited fat synthesis from glucose
Limited loss of glucose in the urine
Regulation blood glucose levels
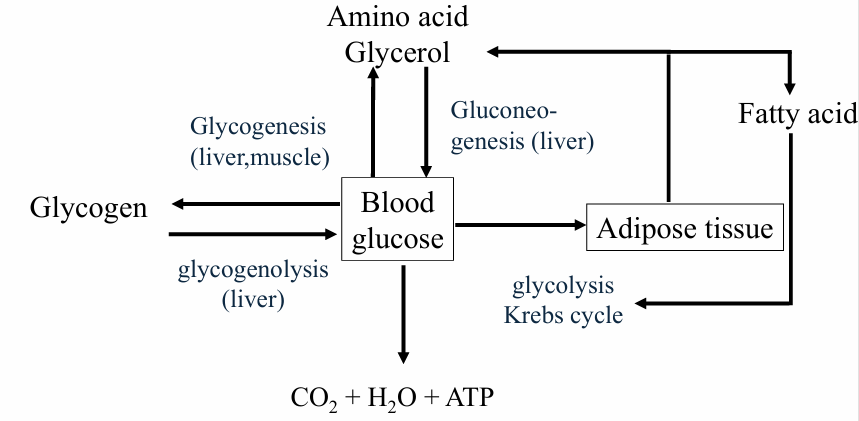

Exceptions
no glucose production from FA!!
Ketone bodies = can be used as alternative energy source
ketosis | ketoacidosis | |
what | Low level of ketones in the blood | Extremely high level of ketones in the blood |
where | mitochondria of liver | |
cause | fasting, exercise, consumption of high-fat diet | (diabetics) |
respons |
| drop in pH of blood → impair the ability of the heart to contract → loss of consciousness |
Carbohydrate metabolism: post-absorptive state vs carbohydrate rich breakfast
post-absorptive state
= last meal has been absorbed from the intestinal tract (e.g. overnight fast)
insulin/glucagon ratio is reduced
glucose enters the blood exclusively from the liver
glycogen → glucose
gluconeogenesis: lactate → glucose
muscle: AA (alanine)→ glucose
adipose tissue: glycerol → glucose
Carbohydrate rich breakfast
glucose concentration ↑
insulin secretion ↑ → insuling/glucagon ratio rises
glucose to tissues
glycogen storage in liver and muscles
anaerobic glucose metabolism: lactate concentration ↑ → glycogen(liver)
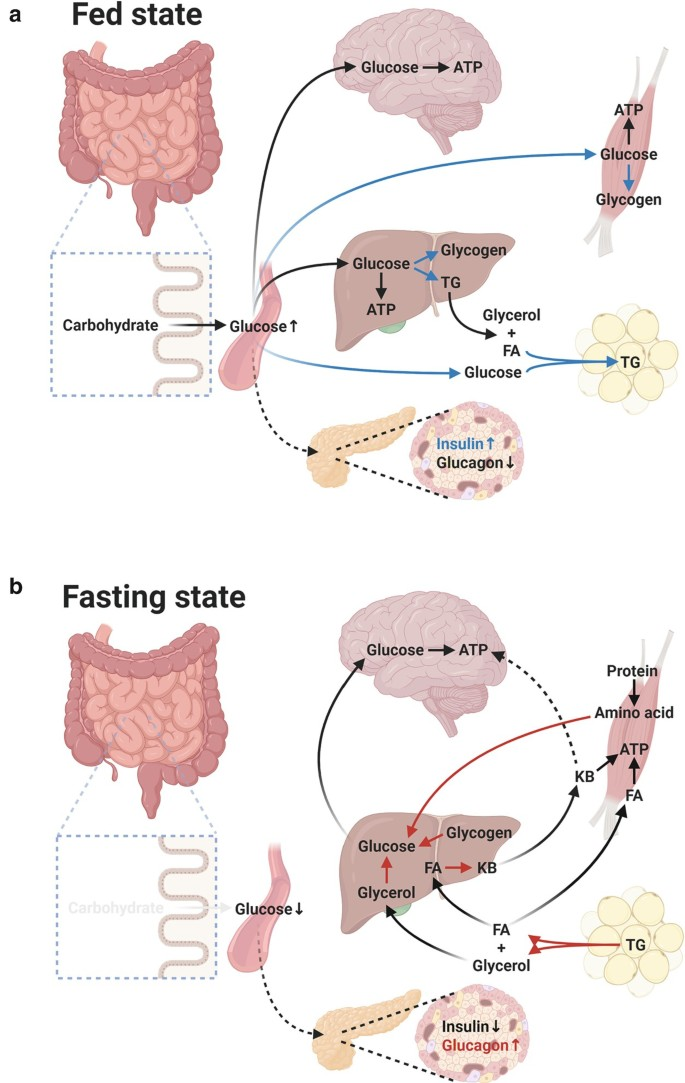


Carbohydrate metabolism: Glycemic vs non-glycemic carbohydrates
Glycemic carbohydrates
Glycemic index (GI)
= measure for the increase in blood glucose after consumption of standard amount of carbohydrate
surface under blood-glucose response curve
calculated relative to standard (glucose/ white bread)
High GI
“fast” carbohydrates that quickly release glucose in the blood
Low GI
'“slow” carbohydrates that release glucose more slowly into the bloodstream
Dependency GI
food characteristics
person-related factors
Non-glycemic carbohydrates
do not directly influence the insulin response (eg. dietary fibre which is not digestible in the small intestine)
Fat metabolism
1) NEFA’s (Non-esterified fatty acids) are carried in the plasma bound to albumin
each molecule albumin has binding sites for about three FAs
2) LP (lipoproteins) transport TAG and cholesterol
core of TAG and cholesterol ester + outer layer of phospholipid and free cholesterol
apolipoprotein = carrier protein
Fat metabolism: different types of LP’s
Type of Lipoproteins | From | To | Transport of … | Name |
VLDL | Liver | Tissues | Triglycerides > cholesterol | / |
LDL | Liver | Tissues | Cholesterol > triglycerides | “bad cholesterol” -> oxidized forms accumulate in the endothelial llining of blood vessels and form a risk for CVD |
HDL | Tissues | Liver | Cholesterol > triglycerides | “good cholesterol” |
Chylomicron | Intestine | Liver | Triglycerides > cholesterol | / |
proteins:
dietary TAG + cholesterol
Fat metabolism: maturation of CM and VLDL
1) Apo E or C-II transferred from HDL to CM or VLDL
2)
Apo C-II = cofactor of lipoprotein lipase → catalyses hydrolysis of TAG in CM and VLDL to FFA’s → pass through capillary wall and enter tissue
prevents uptake of CM and VLDL by liver by covering apo-E and apo-B
with continued residence apo C-II is eventually transferred to HDL, which exposes both apo-E and apo-B
Apo E and Apo B mediate the uptake of CM and VLDL remnants by the liver
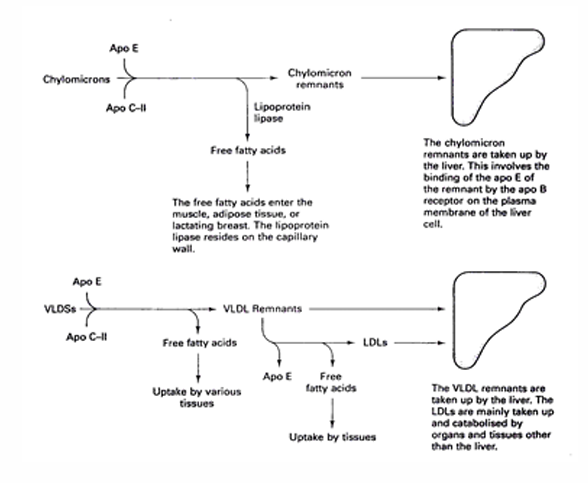
Fat metabolism: NEFA’s after overnight fast and meal
NEFA’s enter plasma from adipose tissue
hormone sensitive lipase: regulates fat mobilization and opposing process
Rate of utilization of NEFAs depend on the plasma concentration of the NEFAs (higher c → higher rate of utilization
plasma NEFA concentration = inverse reflection of plasma glucose and insulin concentration
after overnight fast: insulin and glucose ↓ + NEFA ↑
after meal: shift from fat metabolism to carbohydrate metabolism
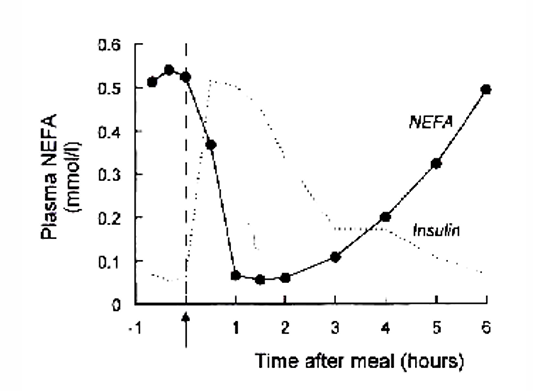
Fat metabolism: post-absorptive state vs after a carbohydrate-fat meal
Post-absorptive state
hormone sensitive lipase (HSL) is activated by low insulin concentrations + adrenaline in plasma + noradrenaline in adipose tissue
rate of NEFA release = regulated by FA re-esterification within the tissue
NEFAs are liberated from adipose tissue and consumed by different tissues (eg. muscles, liver and renal cortex)
in states of low insulin/glucagon ratio → production of ketone bodies in liver is stimulated


After carbohydrate-fat meal
HSL will be suppressed by rising glucose and insulin concentrations
increase adipose tissue glucose uptake
production of G3P and re-esterification of FA within the tissue → increase glycolysis
release of NEFAs from adipose tissue will be suppressed
tissues receive no further supply of NEFAs and switch to glucose utilisation
increased insulin/glucagon ratio → reduces rate of ketone body formation + release in the liver
Significant amount of fat also in meal!!
TAG is absorbed and processed into CM → released in the blood (slower than glucose or AA, so peak is later) → CM escape the liver → insulin stimulates activity of lipoprotein lipase → TAG are removed from CM by adipose tissue, skeletal muscle and heart


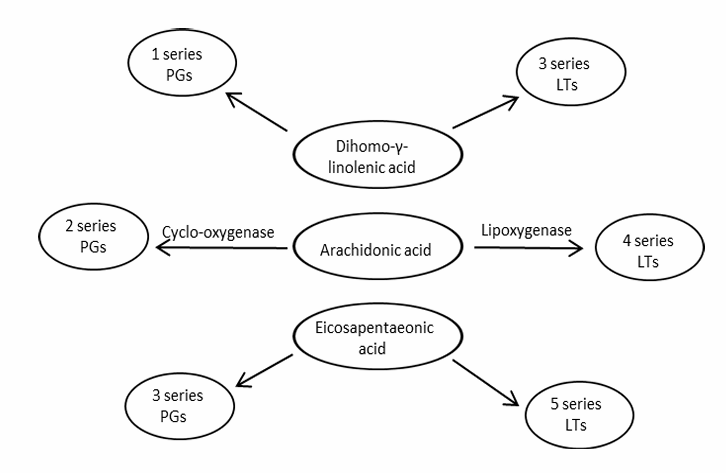
Fat metabolism: Essential fatty acids
animals do not have enzymes to introduce an unsaturated double bond between C-9 and therminal methyl group
a good balance between omega-6 and omega-3
the same saturases and elongases are used
Linoleic acid C18:2\omega6 | Linolenic acid C18:3\omega3 |
desaturase (double bond formation) + elongase (add 2 carbons) | desaturase (double bond formation) + elongase (add 2 carbons) |
AA = Arachidonic acid C20:4\omega6 | EPA = eicosa-pentanoic acid C20:5\omega3 |
→ PG = prostaglandines = mediate inflammation and pain → LT = leukotrienes = regulate function of white blood cells + stimulate contraction of smooth muscles | → PG → LT → DHA = docosa hexanoic acid C22:6\omega3 |

Amino acid and protein metabolism
AA can be oxidized
Functions
Energy store
No fluctuation in e.g. glycogen store
Important locations
skeletal muscle
liver
first organ through which AA pass after absorption
links AA - carbohydrate metabolism
Synthesis of urea

Amino acid and protein metabolism: Measuring turnover of essential amino acids
Turnover = constant cycle of breakdown and replenishmen
1) incorporation of labelled AA (e.g. 13C-leucine)
2) total loss of labeled AA from extracellular pool during steady state = rate of infusion = specific activity x rate of synthesis
Scheme pg 4-16 !!
Amino acid and protein metabolism: transamination (enzyme, what, when to use, examples)
Enzyme
transaminase
What
transfer of an amino group from one AA to a 2-oxo acid
When to use
link between AA and other aspects of metabolism
route for oxidation of AA
Examples
alanine - pyruvate
Aspartate and oxaloacetate
Glutamate and 2 oxoglutarate
Amino acid and protein metabolism: Essential amino acids - approaches
1) Nutritional approach: no synthesis possible at a rate required for normal growth and starting from normally available precursors
Example: Arg when Pro and Gln in the food is low
2) Metabolic apporach: an AA in which a structural unit can not be synthesized via de novo synthesis with human enzymes
Example: Thr
3) Functional approach: an AA that plays a role in maintenance of physiological functions within the body
Example: Phe as precursor for Adrenaline which is responsible for transmitter synthesis
Amino acid and protein metabolism: Non-essential /indispensable AA
can be synthesized de novo via transamination starting from a nitrogen source (ammonia) and a carbon source (alfa-keto acids)
Example: Glu
Amino acid and protein metabolism: Conditionally non-essential /indispensable AA
are synthesized via a more complex pathway
rate of synthesis is limited
Example: cys
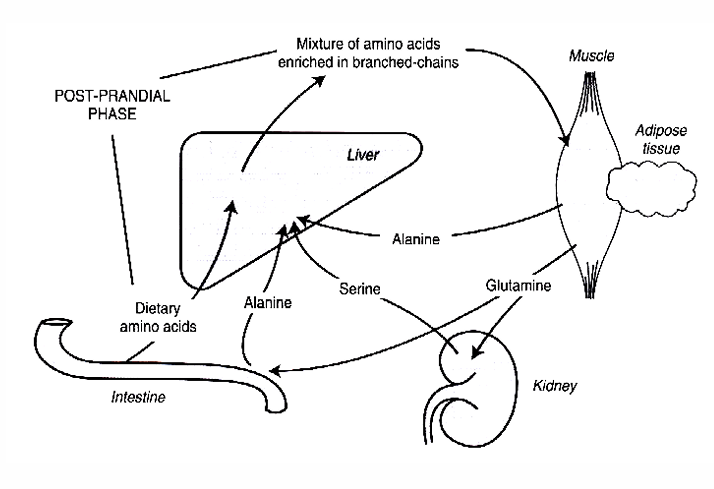
Amino acid and protein metabolism: after a meal - AA interconversion in muscle
1) AA appear in portal vein
2) glutamine ↓ + alanine ↑
3) branched-chains AA leaving the liver (Val, Leu an Ileu) → skeletal muscle
4) Branched-chain AA can be transaminated and oxidized in the muscle → providing a source of energy for the muscle
amino group transferred to 2-oxo-acid
→ pyruvate → alanine
→ 2-oxoglutarate → glutamate
amino groups may form ammonia (at physiological pH) by glutamate dehydrogenase → ammonia + glutamate → (glutamine synthase) → glutamine
Conclusion: catabolism of branched chain AA leads to release of glutamine and alanine
5) Alanine is taken up by the liver for conversion to pyruvate to glucose
6) Glutamine is removed by the kidneys = important metabolic fuel

1. Alanine aminotransferate
2. Leucine, valine or other aminotransferase
3. Glutamine synthase
Glutamate dehydrogenase
Branched-chain 2-oxo-acid dehydrogenase
Muscle protein synthesis
Muscle protein breakdown (= proteolysis)