Fungi- Intro to Fungal Infections; Candidiasis & Cryptococcosis
1/47
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
48 Terms
fungal phylogeny
-over 200,000 genera of fungi
-approximately 200 have been reported to cause human disease
-only about 10 are common causes of infection
fungal infections
-fungi are relatively uncommon causes of invasive human disease- but frequency is increasing
-directly related to the increasing number of patients who are at risk for the development of serious fungal infections: immunosuppressed individuals, invasive medical treatments
opportunistic infections
-infections caused by pathogens that take advantage of opportunities not normally available to them, including:
-altered immunity
-altered microbial flora
-altered anatomy
conditions that can alter host immunity
-general medical conditions: diabetes, cirrhosis, critical illness, nutrition, extremes of age
-autoimmune diseases: lupus, sarcoidosis, etc.
-primary immunodeficiencies: IgA deficiency, CVID, etc.
-cancers: leukemia, lymphomas, etc.
-other infectious diseases: CMV, respiratory viruses, etc.
-HIV infection
-medications and interventions: glucocorticoids, solid organ transplant, bone marrow transplant, chemotherapy, splenectomy, etc.
fungi
-eukaryotic
-have a diameter of 2-10 microns (bacteria ~1 micron)
-fungal cell membranes contain sterols (bacteria do not)
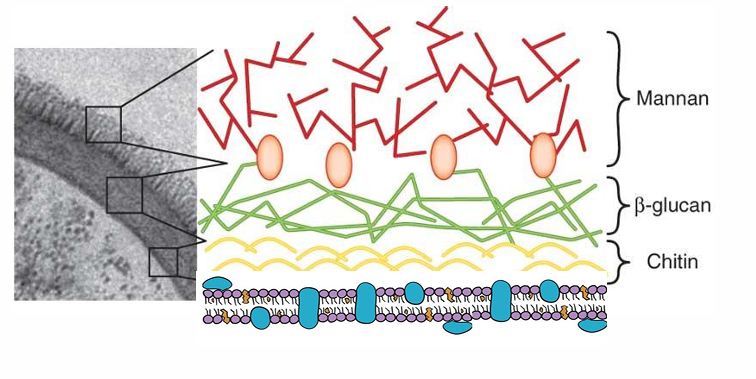
-cell walls contain chitin, glucans, and mannans (bacterial cell walls contain mainly peptidoglycan)
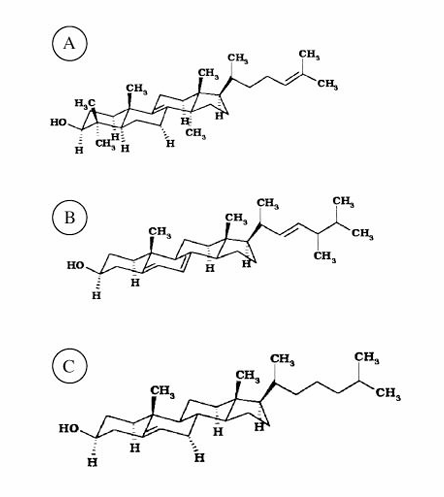
lanosterol, ergosterol, and cholesterol
fungal cell membrane and cell wall
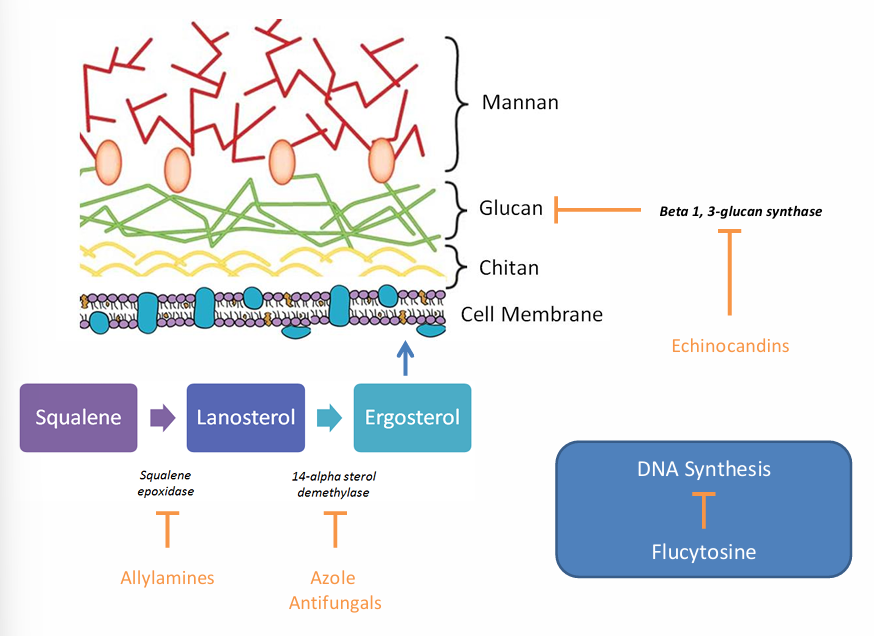
classes of antifungals
-polyenes (amphotericin B, nystatin)
-azoles (fluconazole, itraconazole, voriconazole, posaconazole, isavuconazole)
-echinocandins (micafungin, caspofungin, anidulafungin)
-flucytosine
-allylamines (terbinafine)
allylamines, azole antifungals, flucytosine, echinocandins MOA
fungi can cause
-colonization or infection at various sites (deep or superficial infection; possible sites- sinuses/respiratory, GI, skin)
-can be unicellular (yeasts) or multicellular (molds)
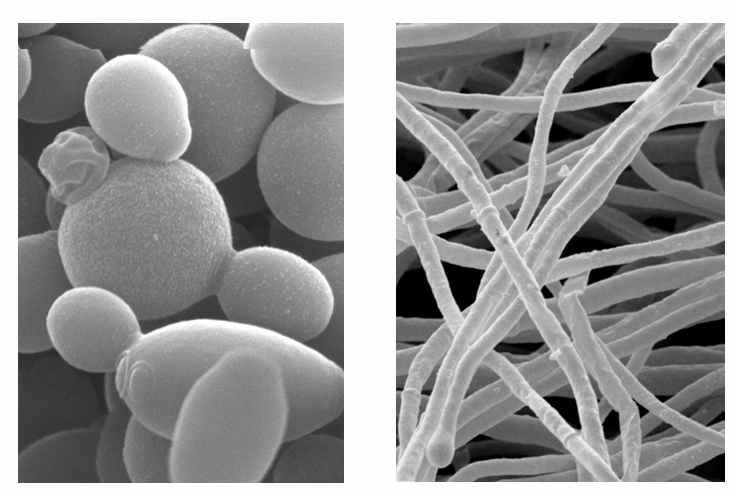
-yeast v mold
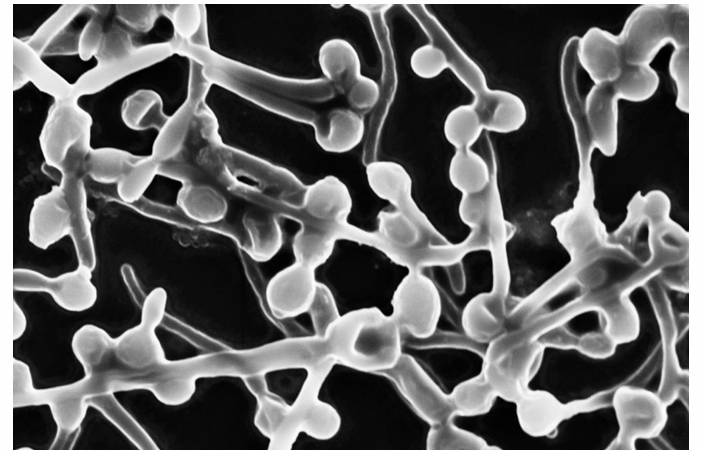
-pseudohyphae
dimorphic (endemic) fungi
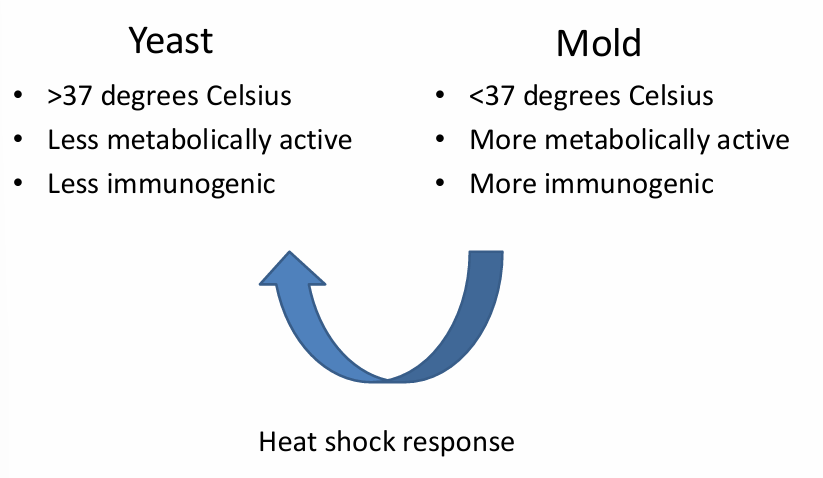
diagnostics
-microscopic observation gold standard: gram stains, KOH preparations, silver stains, immunohistochemical stains
-cultures: variable sensitivity and specificity
-serology
-fungal biomarkers: galactomannan, beta D-glucan (fungitell)
classification
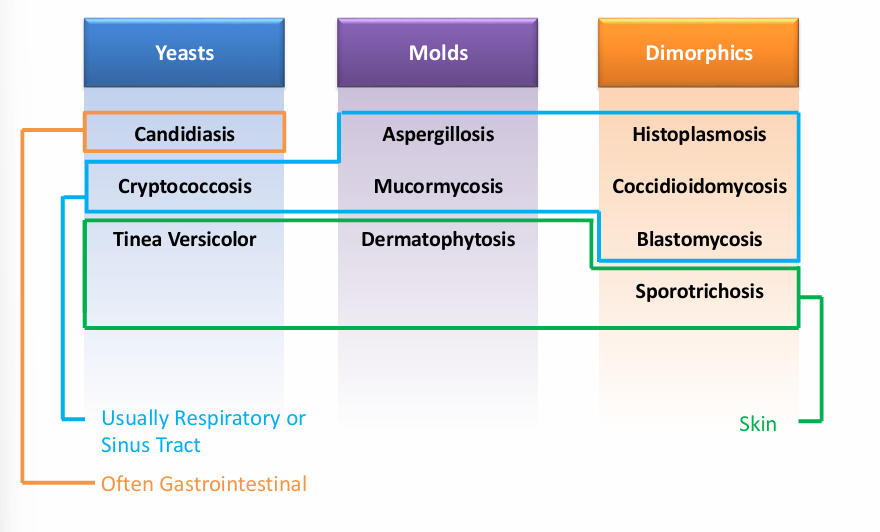
-fungal morphology (yeast v mold v dimorphic)
-host defenses (normal v impaired)
-extent of infection (superficial v mucocutaneous v deep)
-site of primary infection (respiratory/sinus, GI, skin)
overview of fungi morphology
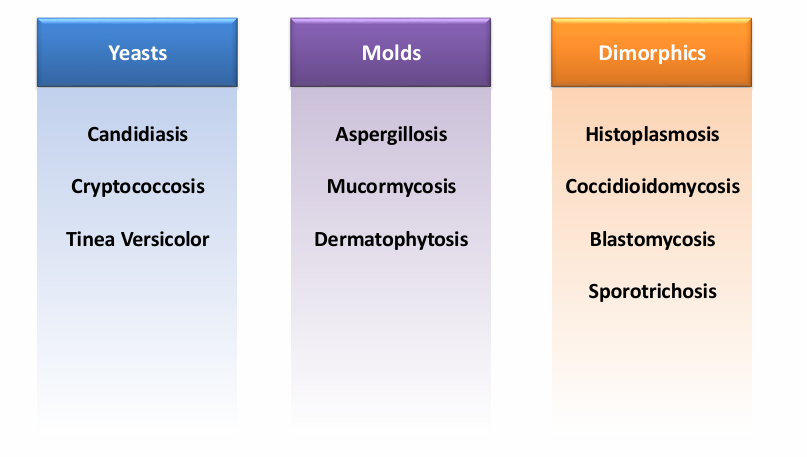
overview of fungi- host defenses
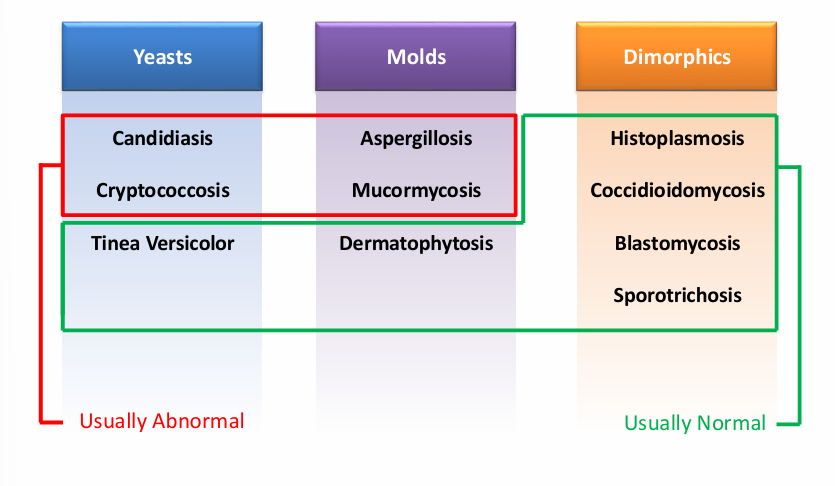
overview of fungi- extent of infection
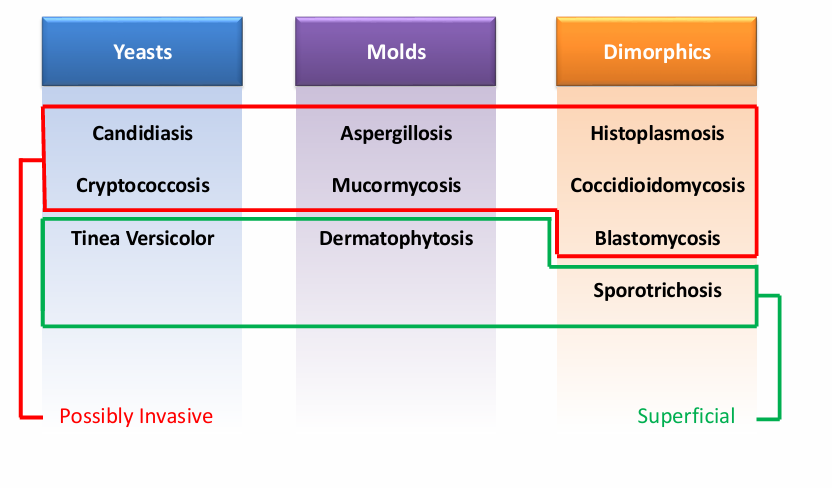
overview of fungi- site of primary infection
candidiasis
-may be divided into mucocutaneous v deep invasive disease
-while it is opportunistic in sense that it requires alteration in normal health, it does not require immunosuppression
-Candida yeast found in normal flora
-visible as small, thin-walled, ovid yeast that reproduce by budding
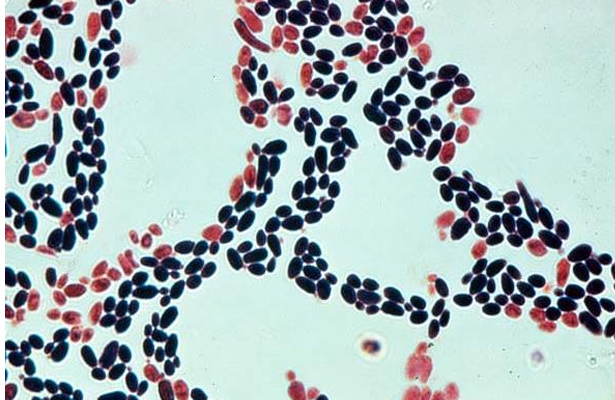
-gram stain, Candida
3 forms of candida
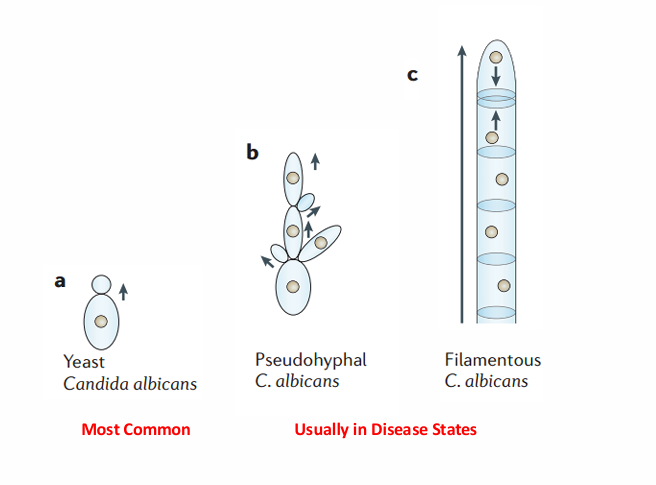
Candida species
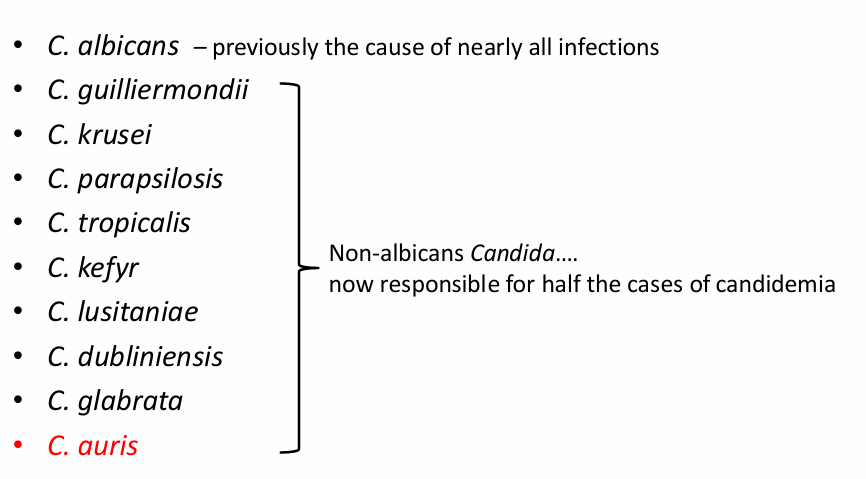
Candida auris
-emerging fungal organism that has been termed a “global public health threat” by the CDC for 3 main reasons:
1) commonly multi-drug resistant
2) can be misdiagnosed by standard microbiology laboratory techniques (reported as C. haemulonii)
3) has caused outbreaks in healthcare facilities due to its efficient colonization of the environment and medical devices
-mortality may be as high as 30%
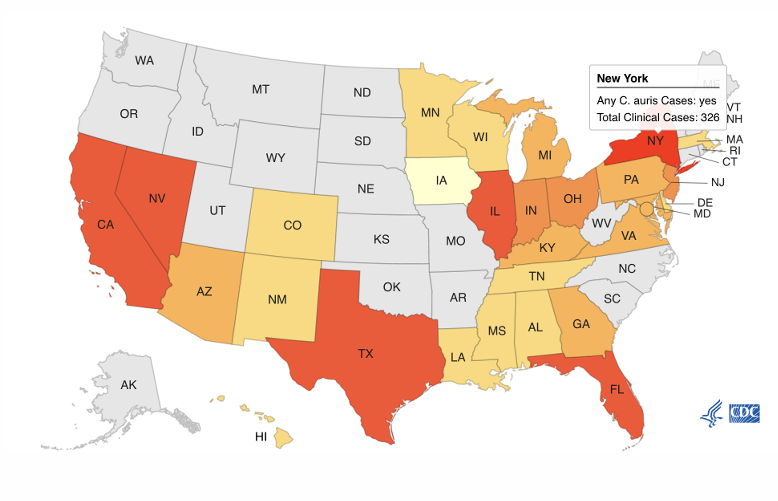
Candida auris epidemiology
risk factors for Candidiasis
-antibiotic use
-disruption of skin/mucosa
-indwelling catheters
-implanted medical devices
-total parenteral nutrition
-surgery
-glucocorticoids
-immunosuppressive agents
-severe burns
-IV drug use
-HIV infection
Candidiasis pathogenesis
-as Candida yeast are part of the normal human microbiome, most infections with the organism arise from our own flora
-disease occurs due to a change in either the organism, the host, or both:
1) change in the host- antibiotic use, disruption of mucosa, or introduction of a catheter/device leads to proliferation of yeast
2) yeast change to hyphal phase: hyphae attach to epithelial cells and the ECM, secrete proteinases and phospholipases, and invade surrounding tissue
3) hyphae disseminate hematogenously to form abscesses in other organs
-C. glabrata does not have hyphal form but can cause disseminated disease
mucocutaneous infections
-oral candidiasis (thrush)
-esophageal candidiasis
-vulvovaginitis
-balanitis
-chronic mucocutaneous candidiasis
-paronychia/onychomycosis
-intertrigo
-erosio interdigitalis blastomycetica
-candida miliaria
-perianal candidiasis and diaper dermatitis
-folliculitis
-generalized disseminated cutaneous candidiasis
-macronodular lesions of candidemia
oral candiadiasis (thrush)
-seen in patients with severe illness, immunodeficiencies (including diabetes), and users of inhaled steroids
-if thrush occurs in healthy individuals, they should be evaluated for underlying immunodeficiencies (including HIV infection)
-oral candidiasis often accompanied by esophageal involvement causing odynophagia, acid reflux, and nausea
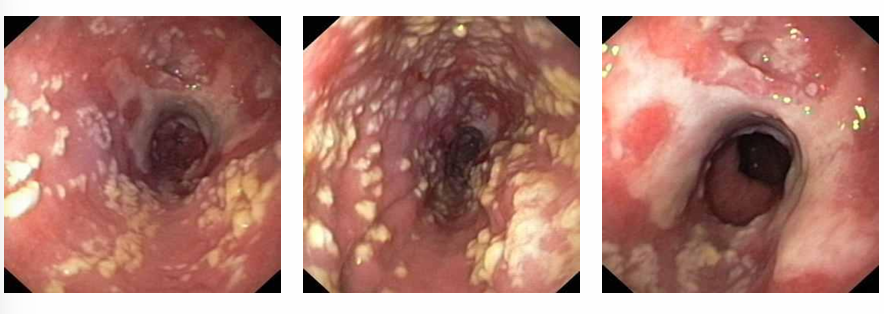
-candidal esophagitis
vulvovaginal candidiasis
-common, lifetime prevalence of 75%
-symptoms: pruritus, pain, thin to “curd-like” white discharge
-often associated with antibiotic use (reduction in bacterial flora resulting in overgrowth of Candida)
-diabetes, immunosuppression, and IUD known risk factors
balanitis
-caused by Candida in approximately 1/3 of cases; more common in uncircumcised men
-causes pain and erythema, may spread to scrotum and surrounding tissue
-pathogenesis is same as vulvovaginal candidiasis (reduction in normal flora with Candida overgrowth)
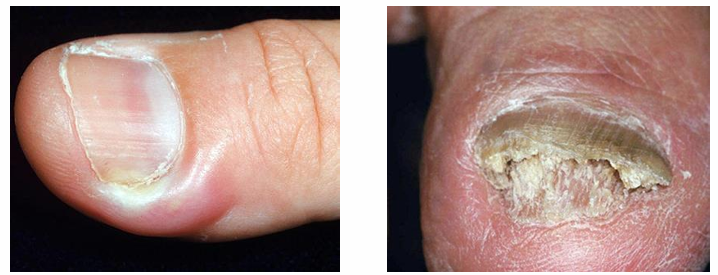
paronychia and Onychomycosis
-paronychia: painful swelling at nail-skin interface
-onychomycosis: infection of nail resulting in white discoloration, nail thickening, and loss
-both conditions are common in individuals who frequently immerse their hands in water
candidal intertrigo
-pruritic eruption with erythematous macerated patches and satellite vesiculopustules
-commonly occur in genitocural, gluteal, interdigital, inframammary, or axillary skin folds
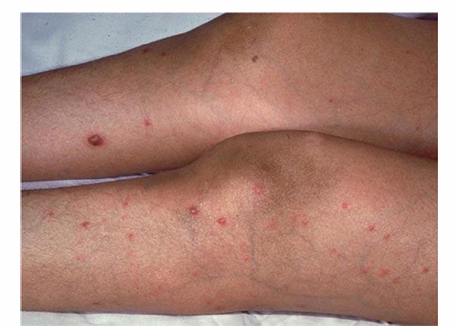
hematogenously disseminated candidiasis
-painful, macronodular lesions
-if present, lesions suggest disseminated candidiasis and the patient should be evaluated/treated for other sites of infection
invasive candidiasis
-presents as a sepsis syndrome
-hematogenous spread most common
-multiple organs can be involved (brain, retina, heart, kidneys, liver, and spleen)
-if hematogenous spread suspected, the eyes should be examined to rule out retinal involvement
-invasive candidiasis can also occur from: contiguous spread through the skin, colonization of a catheter or medical device, GI tract involvement with perforation or erosion of mucosa
diagnosis of Candidiasis
-direct visualization of hyphae: visualization of yeast forms is less helpful as they occur in normal flora
-culture readily available but cannot discriminate between colonization and infection: positive blood cultures always represent real infection; in contrast, positive sputum cultures rarely represent true disease
-biomarkers: Beta-D-Glucan assay (Fungitell) has high negative predictive value for invasive disease (not specific- Aspergillus and PCP also affect the assay)
treatment of candidiasis- mucocutaneous
-cutaneous: topical azole cream
-thrush: nystatin oral liquid/ fluconazole
-esophageal/GI: fluconazole
-vulvovaginal: oral fluconazole v. azole or nystatin suppository
treatment of candidiasis- hematogenous
-usually initiate therapy with an echinocandin (micafungin, caspofungin, anidulofungin)
-azole antifungals (particularly fluconazole) are effective if the isolate is susceptible
special treatment considerations
-C. glabrata and C. krusei sometimes resistant to fluconazole so consider an echinocandin: C. auris is multi-drug resistant (almost always resistant to fluconazole, sometimes resistant to amphotericin) so echinocandins are first-line treatment
-for immunosuppressed patients with invasive disease or instability, start broad and narrow once patients are improving (superficial disease can still be treated with fluconazole)
-try to address any underlying score (indwelling lines, foreign material, etc.)
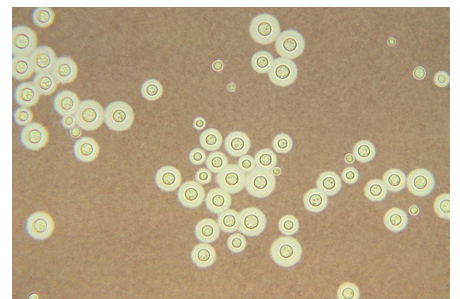
cryptococcosis
-infections caused by yeasts from the genus Cryptococcus
-species: C. neoformans and C. gattii
-appears as large encapsulated yeast
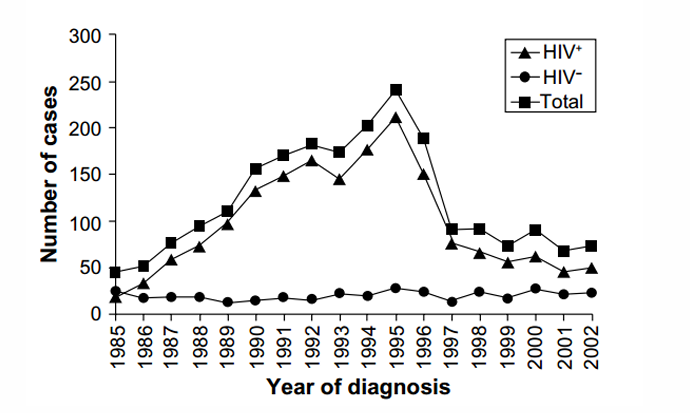
Cryptococcosis epidemiology
-infections occur overwhelmingly in immunocompromised individuals:
-HIV/AIDS
-patients with transplants, hematological malignancies, or those receiving immunosuppression (chemotherapy, biologics, glucocorticoids)
Cryptococcosis epidemiology
Cryptococcosis pathophysiology
-cryptococcus found in soil contaminated with bird droppings, specifically from pigeons (C. gattii not found in soil but found in certain trees, unlike C. neoformans can cause infections in immunocompetent individuals)
-while the pathogenesis of cryptococcosis poorly understood, primary infection occurs when aerosolized conidia enter the lungs
-majority are cleared by host’s immune system; TH1 lymphocytes particularly important in control of initial infection
Cryptococcosis pathogenesis
-local environment of the lungs signals C. neoformans to transform into its yeast form
-yeast’s capsule is anti-phagocytic
-melanin is produced which blunts the host’s immune response
-cells divide and multiply by budding
-subsequently disseminate through the bloodstream, both intracelluarly (macrophages) and extracelluarly
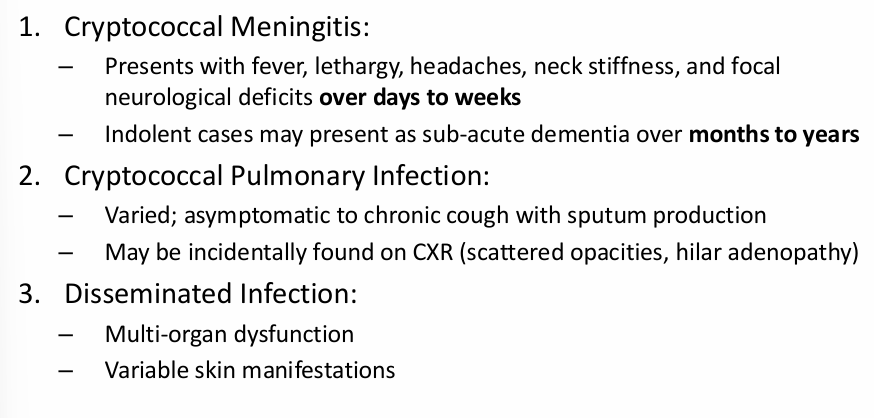
Cryptococcosis clinical manifestations
Cryptococcosis diagnosis
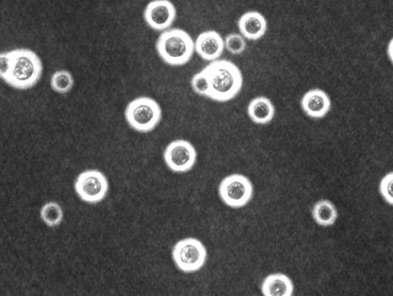
-histopathology is gold standard
-India ink CSF stains approximately 50% sensitive for CNS disease
-CSF studies will often reveal a mononuclear or lymphocytic pleocytosis, elevated protein, increased opening pressure, and normal glucose
-cultures are nearly 100% sensitive for CSF and 70-80% sensitive for blood but take several days to return
-cryptococcal antigen tests for blood and CSF are both sensitive (>90%) and specific (>90%); beta-D-glucan is negative
-cryptococcal nucleic acid amplification also commonly used
-MR imaging may reveal leptomeningeal enhancement or cryptococcomas
Cryptococcosis treatment
-CNS Cryptococcosis: amphotericin B + flucytosine followed by long-term fluconazole
-pulmonary cryptococcosis: mild-to-moderate- fluconazole, severe- amphotericin B followed by fluconazole, echinocandins have no coverage of Cryptococcus