Lecture 9 - Thyroid Hormones and Drugs, Gonadal Steroid Hormones and Drugs
1/61
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
62 Terms
Describe the functions of Thyroid Hormones
Responsible for metabolism, energy uptake
growth and development
maintains homeostasis in nearly every tissue and organ
Where are thyroid hormones synthesized?
in the Anterior Pituitary (AP) in the Hypothalamus (Hypothalamic-Pituitary-Thyroid Axis)
Describe how Thyroid Hormones Synthesis is Regulated
Thyrotropin-releasing hormone (TRH) is synthesized by neurons arising from the hypothalamus, which release TRH into the hypophyseal portal system
TRH activates its own set of GPCRs in thyrotrophs which stimulates the release of thyrotropin (thyroid-stimulating hormone TSH) in the anterior pituitary
TSH binds GPCRs on the cell surface of thyroid cells, which activates Adenylyl Cyclase, which in turn increases production of cAMP
cAMP and Ca2+ are utilized as 2nd messengers which stimulates the production and growth of thyroxine (T4) and triiodothyronine (T3) - thyroid hormones
Once T4 and T3 reach their target tissues, they will stimulate nuclear hormone receptors (thyroid hormone receptors) to regulate transcription of different genes
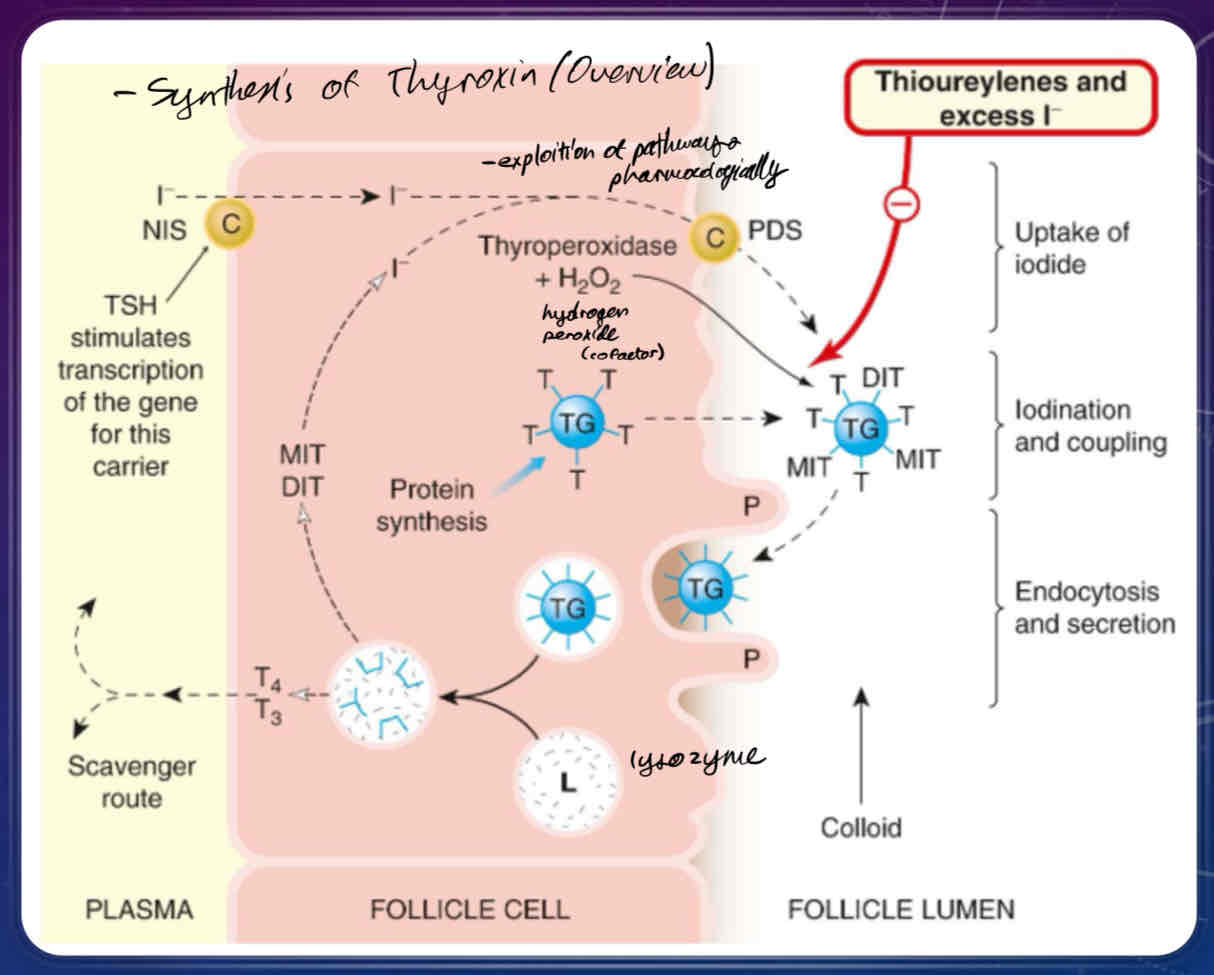
Describe the cascade of events behind thyroid hormone synthesis once TSH binds to specialized cells of the thyroid gland
iodide is incorporated into tyrosine residues to make thyroid hormone
upon stimulation of the TSH receptors and changes of intracellular Ca2+, a sodium iodide transporter protein will be activated, bringing iodide and sodium into the cell
iodide ions combines with thyroperoxidase +hydrogen peroxide, which allows thyroglobulin-bound tyrosines to be incorporated into thyroglobulin
thyroglobulin is coupled with many molecules of tyrosine, which are transported to the lumin of the follicle
produces monoidodinated and diiodinated thyronine, which are coupled together to produce T3 and T4
thyroglobulin molecule is taken back up by endocytosis, where it is packaged into lysosomes, where they then fuse with the extracellular membrane to release T3 and T4 into the bloodstream.
Describe the ratio of T4 to T3
most physiological thyroid produced is T4
only a tiny fraction (1/2000 T4 and 1/200 T3) is free (ie available to work)
only free T4 and T3 is used to gauge thyroid gland function
since T3 is 3 to 5 times more potent than T4, T4 can be metabolized to T3 in the thyroid and periphery by de-iodination of the third carbon of the external benzene ring of T4
if internal benzene ring of T4 is de-iodinated, it produces a physiologically inactive form of T3 (reverse T3) that acts as a competitive inhibitor of T3 at the thyroid receptor
What is Protirelin?
TRH receptor agonist (TRH replacement)
used for diagnoses of Hypothalamic Insufficiency (a type of thyroid insufficiency)
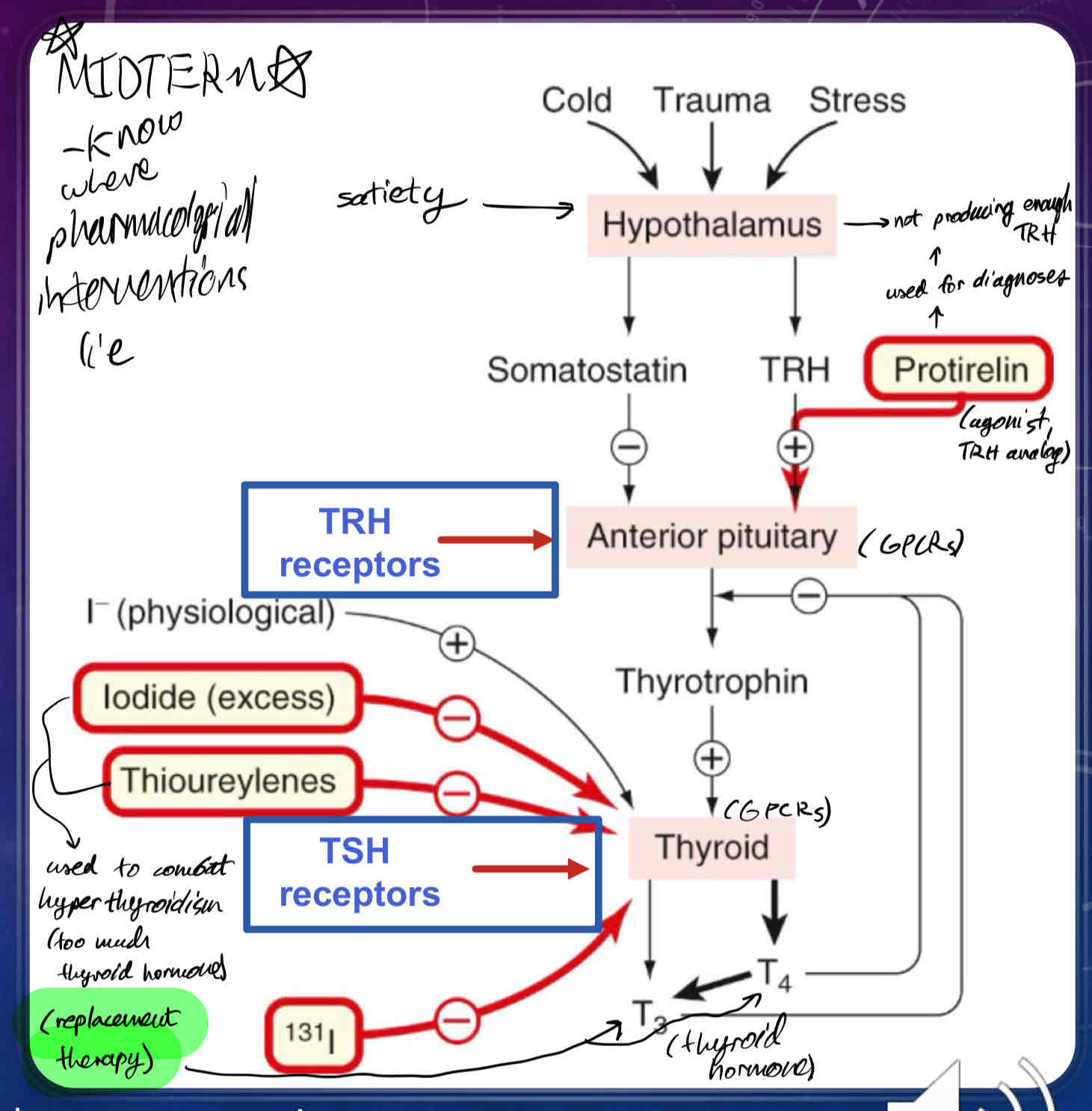
What does Somatostatin do?
inhibits secretion of TSH (thyroid-stimulating hormone) from anterior pituitary
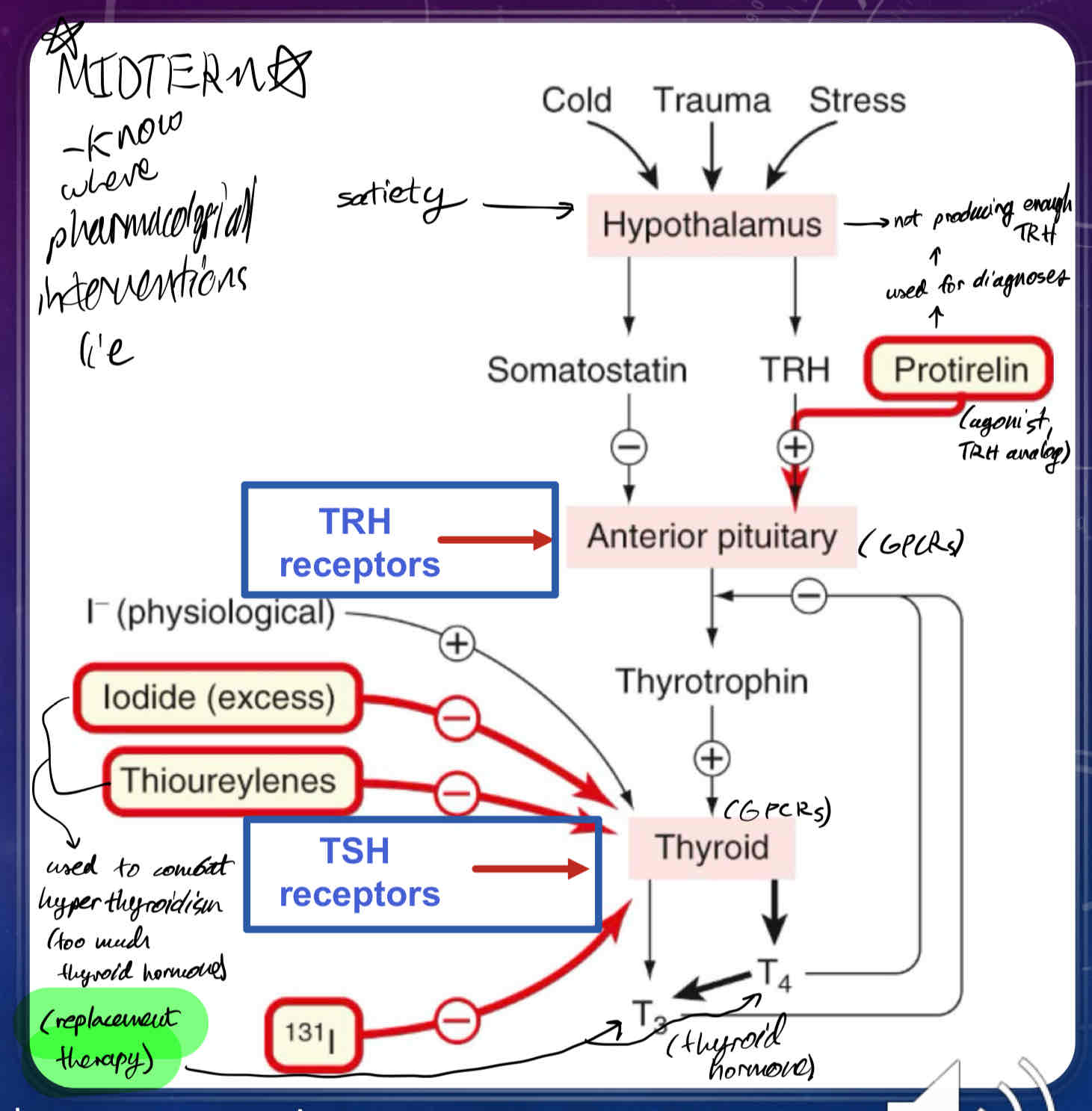
What does naturally occurring (physiological) Iodide do?
promotes thyroid hormone synthesis
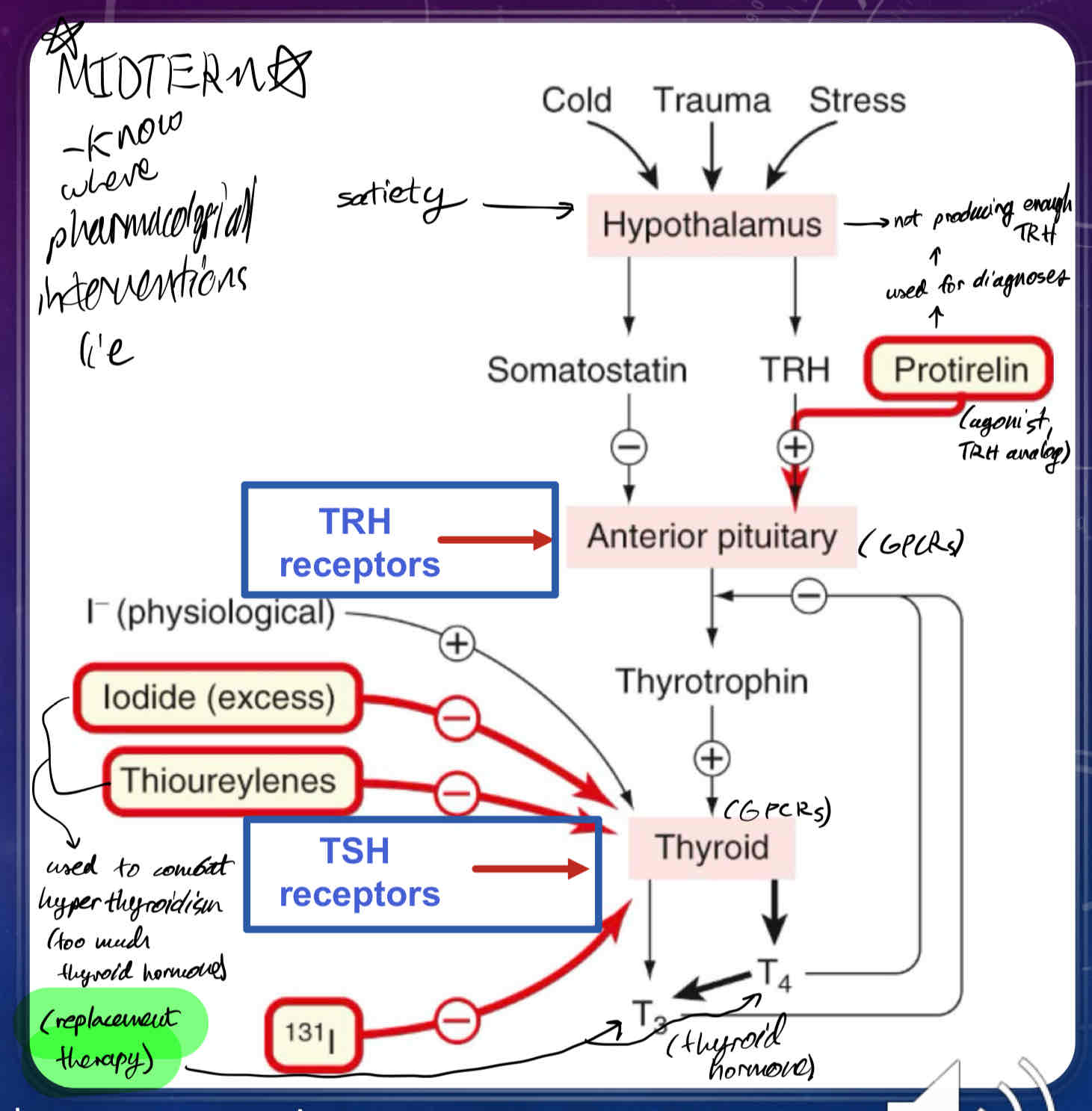
What does excess/exogenous Iodide (30x requirement), and Thioureylenes do?
can be used to suppress thyroid hormone function via thyrotoxicosis
What does radioactive iodide (131I) do?
used for Thyroid cancer
used to oblate the thyroid
the thyroid preferentially takes up iodide, whereas most cells in the body do not.
when someone is given radioactive iodide, it gets concentrated in the thyroid gland to kill the cells of the thyroid gland.
rest of the free radioactive iodide is regularly excreted in the urine
What results in Goitres?
Overproduction of T4 Thyroid hormone
Describe the symptoms of Hypothyroidism
impaired skeletal growth
impaired growth and development of CNS
impaired protein synthesis and carbohydrate absorption (metabolism in all tissues)
impaired adrenocortical, gonadal, cardiac, renal, and liver functions
What is Hashimoto’s Disease?
A disease resulting from Hypothyroidism
Auto-immune disorder where the body produces antibodies to the thyroid gland, inhibiting synthesis of T3 and T4
What is Congenital hypothyroidism?
A condition of severely stunted physical and mental growth due to untreated thyroid hormone deficiency at birth
How is hypothyroidism treated?
Replacement Therapy
administration of Liotrix***
4:1 combination of T4 to T3
mimics physiological concentration in the blood
can be titrated in the blod
drug of choice for treatment
synthetic Levothyroxine (L-T4) is administered
has a long halflife
high bioavailability
first-line approach
synthetic L-triiodothyronine (Liothyronine / L-T3)
more potent and faster onset than L-T4
shorter duration of action (reserved for acute emergencies)
What two conditions together generally result in the removal of the thyroid?
Thyroid cancer
Hyperthyroidism
What is Graves Disease?
Results from Hyperthyroidism
auto-immune disease where an antibody (TSIs) is sent to the TSH receptor and activates it, causing high levels of circulating T4
causes goitres, bulging eyes
common in middle-eastern populations
4x more prevalent in women than men
What is neonatal hyperthyroidism?
antibodies (TSIs) cross placenta and activate T4 production in fetus
T4s cross back into maternal blood system and are very soluble
requires quick treatment
How is Hyperthyroidism treated?
Inhibition of thyroid hormone synthesis
propylthiouracil (PTU) blocks iodination of tyrosine and the coupling of iodotyrosine to thyroglobulin
Inhibition of Thyroid hormone release - excess iodide ion (30x physiological concentration) prevents thyroid hormone release from thyroglobulin by inhibiting lysosomal proteases
used for treatment of thyroid storm in neonatal hyperthyroidism
Ablation of the thyroid gland - radioactive iodine (I131) is rapidly taken up by the thyroid and the radioactivity kills thyroid cells
used for thyroid cancer
used for residual cancer cells after surgical removal or cancerous thyroid cells.
What are the Gonadal and Gonadotrophic hormones responsible for?
Sex hormones
conception
embryonic development
development at puberty
desire and ability to procreate (arousal, fertility)
How does the hypothalamus control gonadotrophs (sex hormones)?
Gonadotrophin releasing hormone (GnRH) is produced in cell bodies of neurons originating in the hypothalamus
GnRH reaches the anterior pituitary via the hypophyseal portal system and binds GPCRs on gonadotrophs - specialized cells that produce gonad stimulating hormones
stimulates Phospholipase C to act, increasing intracellular Ca2+ concentrations
stimulates release of two gonadotrophins
leutinizing hormone (LH)
supports growth of the follicle in ovarian cycle
follicle-stimulating hormone (FSH)
induces ovulation and supports growth of corpus luteum
Describe the Ovarian Cycle
a continuation of hypothalamic control of gonadotrophs
GnRH release is pulsatile and targets GPCRs on the anterior pituitary
days 1-14 of menstruation cycle are the follicular phase, where FSH is released into the ovary
days 15-28 are the luteal phase where LH is released into the ovary
Anterior pituitary releases both FSH and LH into the ovary, producing follicles
follicles rupture at cortex of ovary, releasing an ovum
remainder of follicle degenerates, producing corpus luteum
produces progesterone, which is involved in maintenance of the endometrium and negative feedback of anterior pituitary AND hypothalamus
if LH produced is greater than FSH produced, follicle will produce estrogen
if FSH produced is greater than LH, corpus luteum will produces progesterone
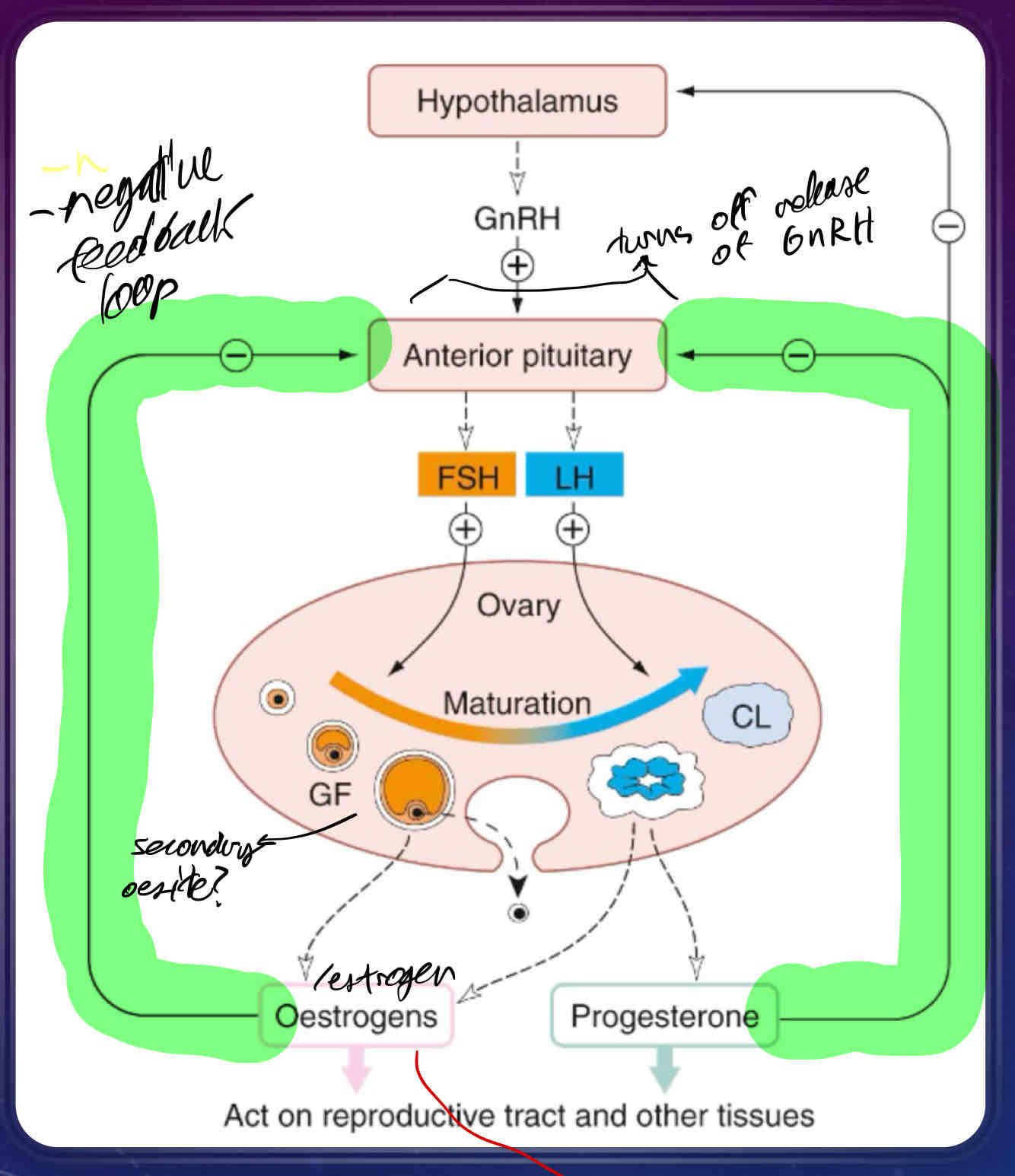
What is the proliferative phase of the ovarian cycle?
estrogen is at it’s peak to maintain endometrial regeneration
responsible for rupture of the follice/release of ovum
endometrium increases in thickness and vascularity
estrogen binding to endometrium causes endometrium to produce progesterone receptors, making endometrium sensitive to progesterone
estrogen stimulates cervix to produce a protein/carb rich mucus to facilitate entry of sperm
Luteinizing Hormone concentration in the plasma spikes just prior to secretory phase
stimulates rupture of follicle
stimulates development of corpus luteum
What is the secretory phase of the ovarian cycle?
progesterone is secreted by corpus luteum and promotes growth of endometrium to facilitate implantation of fertilized ovum
if implantation of fertilized ovum doesn’t occur, corpus luteum will degenerate, and production of progesterone will stop, leading to menstruation
if implantation of fertilized ovum does occur, the ovum will secrete human chorionic gonadotrophin (HCG) which maintains lining of uterus during pregnancy
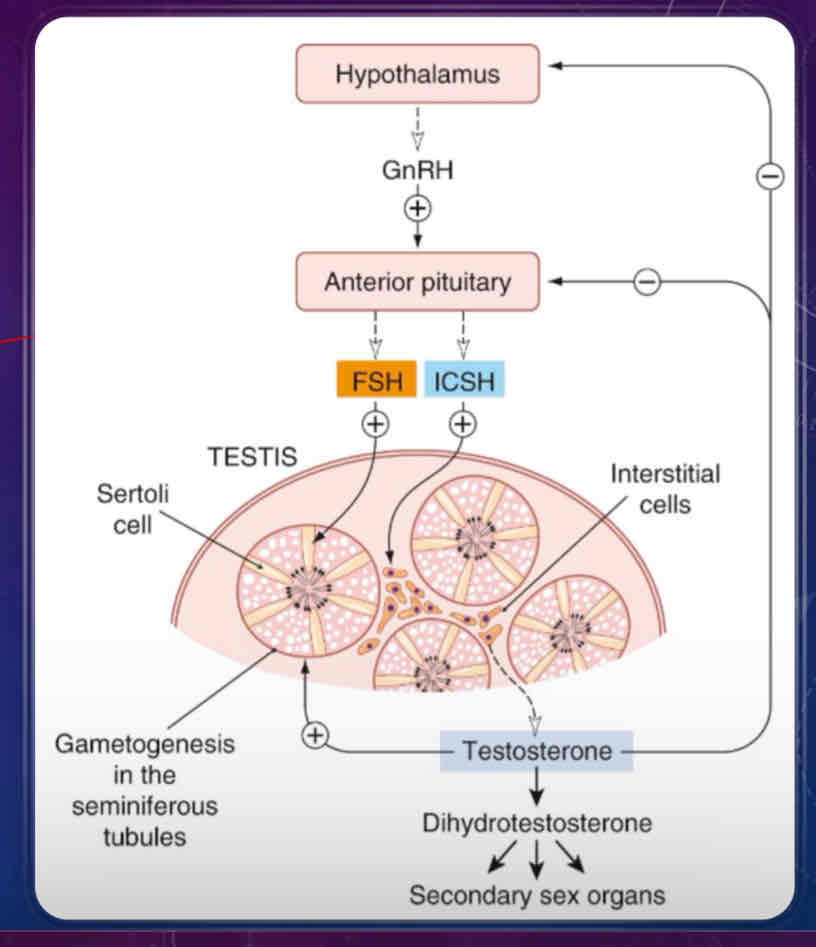
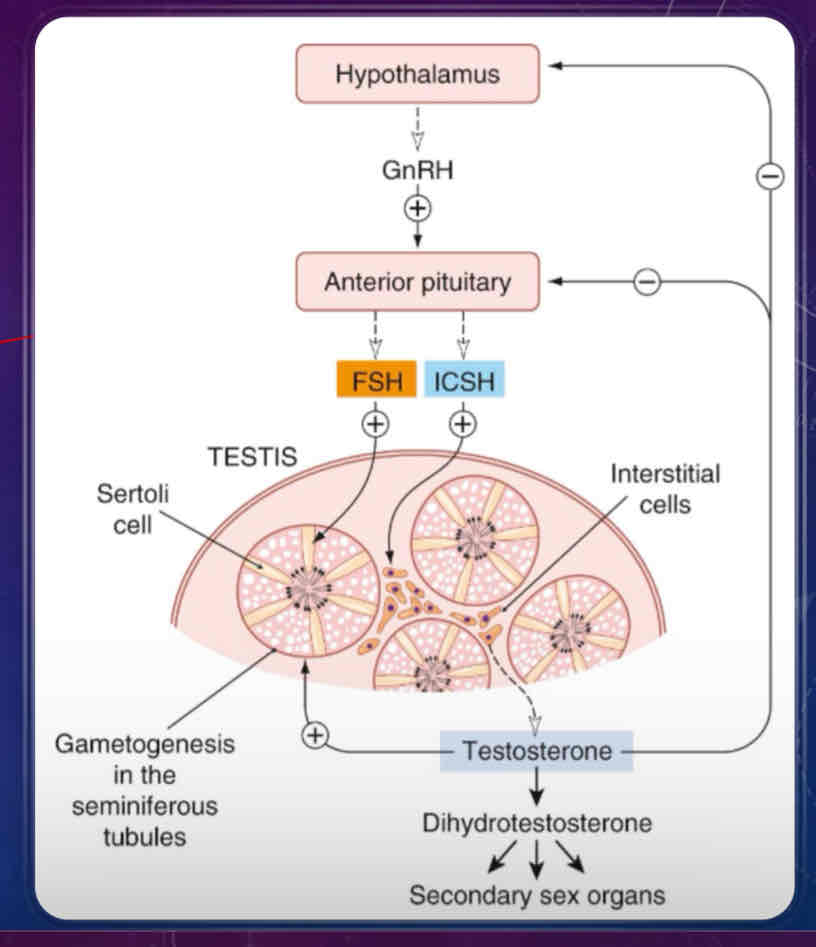
Describe the male reproductive cycle
similar to ovarian cycle, it’s controlled by the hypothalamus, which release GnRH onto the gonadotrophs of the anterior pituitary
stimulation of the anterior pituitary will also release FSH and interstitial cell-stimulating hormone (ICSH), which is the same as LH in females
ICSH stimulates production of testosterone from interstitial cells
has an effect on primary and secondary sex characteristics
FSH and testosterone maintains seminiferous tubules which support sperm production (gametogenesis) through Sertoli cells
testosterone and dihydrotestosterone are secreted into the blood are also responsible for secondary sex characteristics (eg body hair)
What is the result of abnormally high FSH/LH Levels in the blood?
infertility/sub-fertility
normal in women who have recently undergone menopause
abnormal in normal reproductive years, can be a result of
premature menopause
poor ovarian reserve
gonadal dysgenesis
Turner Syndrome (females with only 1 X chromosome)
Castration
testicular failure
What is the result of abnormally low FSH/LH levels in the blood?
infertility
failure in gonadal function (hypogonadism)
in males: low levels of sperm and/or testosterone produced
in females: cessation of reproductive cycle
Polycystic Ovarian Syndrome
high LH, low FSH
associated with higher male hormone levels in women
physical signs in secondary sex characteristics
Hypothalamic suppression and Hypoptuitarism: individuals who have this before puberty do not go through puberty
Gonadotrophin deficiency
gonadal suppression therapy - use because of gonadal tumours
GnRH antagonist
GnRH agonist
What can be used to treat abnormal levels of FSH and LH?
Human Chorionic Gonadotropin (HCG)
very similar to LH
induces ovulation in females
stimulates testosterone/sperm production in males
Menotropin - a combination of LH and FSH used to mimic LH surge
Follitropin - recombinant FSH
used to stimulate ovulation and fertility
Lutropin - recombinant LH
used to stimulate ovulation and fertility
How is estrogen synthesized?
derived from cholesterol (like all steroidal hormones)
undergoes high serum binding (like thyroid hormone); only free fraction is “free” (physiologically available)
large pool of circulating estrogen that is responsible for more than sexual arousal/fertility.
response to external stimuli
waking up
maintenance of bones
central effects
binds estrogen receptors intracellularly
2 subtypes of estrogen receptors
alpha
beta
membrane-bound GPCR (recently discovered)
receptors are transcription factors
regulate gene expression
What are the roles of Estrogen?
growth and development of female sex organs
development of secondary sex characteristics
prevention of bone reabsorption/osteoporosis (failure of calcium mobilization)
sexual and maternal behaviour
sensitization of tissues to progesterone; estrogen turns on progesterone receptors, activating progesterone gene transcription
negative feeback regulation of gonadotrophs
How is estrogen metabolized?
estrogen (17beta-estradiol/E2) is not very water soluble, so it must be metabolized to estrone and estriol, which are hydrophillic entities, to be excreted
also weak agonists of estrogen receptor (therefore more readily excreted)
estrogen conversion to E2 is reversible, so it is in equilibrium with E2
What are the three clinical uses of estrogen analogs?
Hypogonadism - replacement therapy in females who do not produce enough estrogen
Contraception - used in combination with progestogens to inhibit production and release of GnRH, LH, and FSH, preventing ovulation
Morning After Pill - high doses of ethinylestradiol taken shortly after sex
hastens passage of ovum through fallopian tube through withdrawal bleeding, preventing embryo implantation
Oral Contraceptive
Combined Pills (estrogen and progestogen)
taken 21 of 28 days
estrogen inhibits FSH release, progesterone inhibits LH release
Progestogen-only pills
taken continuously
less reliable but taken by people at high risk for estrogen-sensitive breast cancer
Postmenopausal Hormone Replacement Therapy - prevents vasomotor instability (hot flash), headache, osteoporosis
What are some synthetic preparations of estrogen and what are they used for?
found in contraceptives
17beta-estradiol - used in a dermal patch, topical cream, tablet
used for gonadal insufficiency, post-menopausal syndromes
Ethinylestradiol - found in oral contraceptive
ethinyl substitution on one of the carbons of 17beta-estradiol that makes it orally active
Mestranol - first synthetic oral contraceptive, converted to ethinylestradiol in liver
What are some adverse effects of estrogen analogs?
usually resulting from prolonged use, increasing the incidence of:
coronary heart disease due to calcium mobilization
breast cancer since breast tissue is estrogen sensitive
uterine cancer
embolism (blockage of blood vessel)
What are some Estrogen receptor antagonists and what are they used for?
Clomiphene - fertility drug
blocks negative feedback of estrogen, increasing release of GnRH, LH, FSH
Tamoxifen - treatment for breast cancer
mixed agonist/antagonist
Raloxifene - treatment for osteoporosis and reduces risk of invasive breast cancer in postmenopausal females
antagonist for ER-beta, weak agonists for ER-alpha
Where is progesterone produced?
primarily in corpus luteum
additionally in placenta, tests, adrenal cortex
What are the physiological effects of progesterone?
effects mediated through progesterone binding to its receptors in the affected tissues
in the uterus, progesterone is secreted by corpus luteum, which transforms the endometrium into a tissue that allows for implantation of a fertilized ovum
in the hypothalamus and pituitary, progesterone inhibits secretion of GnRH and gonadotropins thus preventing further follicular maturation and ovulation, which is needed for pregnancy to proceed
What are some examples of progesterone analogs/progestogens and their clinical uses?
Levonorgestrel and medroxyprogesterone - progesterone analogs
uterine bleeding
endometriosis (abnormal endometrial proliferation)
metastatic cancers of the endometrium and breast
amenorrhea (absence of menstruation)
birth control (causes amenorrhea)
Mifepristone - progesterone receptor partial agonist
abortion/medical termination of pregnancy
What is the role of Androgens?
development of male reproductive organs during puberty
development of secondary sex characteristics (hair distribution, voice)
somatic development (bone structure, skeletal muscle)
Describe testosterone fate in testis
produced in Leydig cells upon stimulation by LH/ICSH
converted to active metabolite dihydrotestosterone (DHT)
bind the androgen receptor (AR) a nuclear hormone receptor transcription factor
Describe Androgen Synthesis
starts with cholesterol (like other steroid hormones)
androstenedione is a precursor produced either directly from cholesterol or from progesterone
androstenedione is further converted to either testosterone or to estrone by assistance of aromatase enzyme
estrone can be reversibly converted to estradiol (estrogen)
aromatase can also convert testosterone to estradiol (estrogen)
5alpha-reductase can convert testosterone in the plasma to dihydrotestosterone, which is a powerful, active androgen in the body
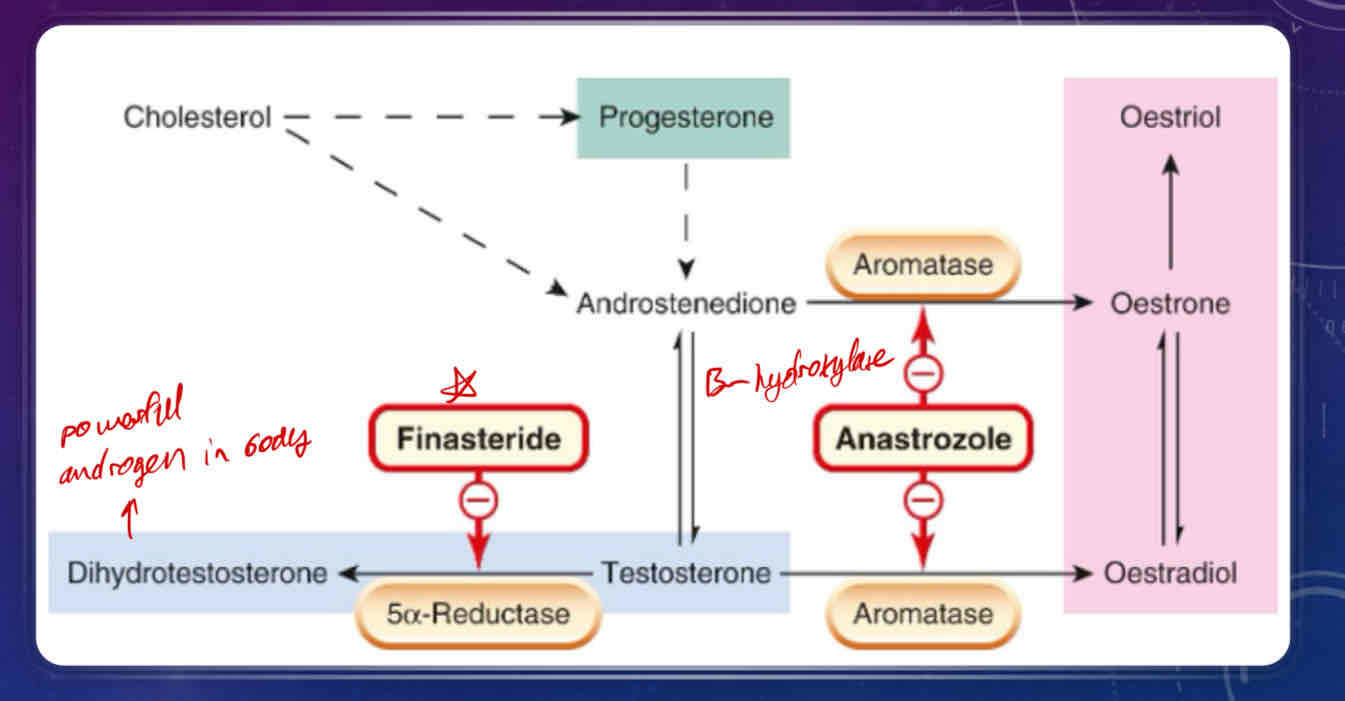
What is Anastrozole?
Aromatase inhibitor
used in androgen therapy for the treatment of estrogen-positive breast cancers
What is Finasteride?
anti-androgen
used for treatment of androgen-sensitive prostate cancer
inhibits testosterone conversion to dihydrotestosterone/ inhibits 5alpha-reductase
Describe the illicit effects of Testosterone and its analog(s)
Anabolic effects
increased bone density/mass
increased muscle mass
increased metabolism (increased uptake of AA’s and carbs by muscle cells)
reduced recovery time
Androgenic effects
reproduction
development at puberty
secondary sex characteristics (abuse is obvious)
Pure Testosterone - readily absorbed through GI tract but subject to first-pass metabolism, therefore is administered usually through transdermal patches or subcutaneously
Nandrolone - synthetic androgen primarily used for metabolic effects
doping in sports
What are some clinical uses of Testosterone analogs?
Androgen Replacement Therapy
Testicular hypotfunctin, pituitary failure, testicular cancer
Anabolics
illicit use among athletes for increasing bone or muscle mass
Female Hyposexuality following ovariectomy
restores plasma testosterone to normal female concentrations and improve sexual function
What are some anti-androgens and their clinical uses?
Cyproterone - Direct androgen receptor (AR) Antagonist
used to treat early puberty in males (precocious puberty)
used to treat masculinization and acne in women
decreases libido and can be used to treat hypersexuality in male sex offenders
Flutamide - Direct AR Antagonist
used to treat prostate cancer
must also be administered with GnRH agonist or antagonist because it blocks negative feedback exhibited by testosterone thus leading to increased LH release
Finasteride - indirectly inhibits androgen function by blocking alpha-reductase so testosterone cannot convert to dihydrotestosterone
used to treat benign prostatic hyperplasia
used to treat prostate cancer
Enzalutamide - AR antagonist
used for metastatic, castration-resistant and castration-sensitive prostate cancer, non-metastatic castration-resistant prostate cancer
Describe how contraction of the non-pregnant uterus works
Myometrial cells are smooth muscle cells in the uterus that act as pacemakers that mediate a tonic contraction of non-pregnant uterus
can produce action potentials that are regulated by female sex hormones
non-pregnant uterus then goes through a pulsatile cycle of contractions that increase in strength as the cycle progresses
Sympathetic neurons affect uterus contraction
Adrenaline inhibits uterine contraction
Noradrenaline stimulates contraction
What does pregnancy cause in the uterus?
increased amount of estrogen made during pregnancy hyperpolarizes (making a membrane potential more negative) myometrial cells so that there is no action potential forming and thus no contractions.
estrogen stimulates expression of oxytocin receptors in the uterus, causing the uterus to become sensitive to the peptide hormone oxytocin
Describe the synthesis and transport of Oxytocin
most produced in neurons arising from the paraventricular nuclei of the hypothalamus
these neurons project into the posterior pituitary via the hypophyseal tract
they terminate in the areas of the posterior pituitary that are rich in vascular beds
thus, oxytocin is taken up freely in the bloodstream and circulates throughout the body
What are the two main effects/uses of oxytocin?
Milk Ejection - stimulates the contraction of myoepithelial cells surrounding glandular ducts that contain milk
occurs after parturition (immediately after childbirth); cells become sensitized to oxytocin and oxytocin levels increase (postpartum effect)
suckling from the baby causes physiological stimulation for oxytocin release
oxytocin can be administered to stimulate milk ejection if there is milk “let-down” impairment (where milk does not fill glandular ducts of breasts)
Uterine Contraction - facilitation (not initiation) of labour
stimulates peristaltic contraction in the uterus and causes cervix to relax
third-trimester pregnant uterus is very susceptible to oxytocin because there are tons of oxytocin receptors on the smooth muscle cells of the uterus
oxytocin given very slowly via IV can be used to stimulate labour contractions (too quick administration of oxytocin can cause hypoxia of the fetus) or to “clamp” the uterus down (to control postpartum bleeding)
What are Ergot Alkaloids and some examples?
uterus stimulators that are derived from a fungus that grows on wheat and grains
vasoconstrictive effects (can cause gangrene)
Ergometrine - highly selective for oxytocin receptors in the uterus
causes strong contractions in BOTH pregnant and non-pregnant uterus
has been previously used to induce abortion, but very dangerous
can be used to treat postpartum bleeding and hemorrhage
can be used to treat migraines due to vasoconstrictive effects (although there are far better drugs to treat migraines with)
How does Prostaglandin E2 and its analogs stimulate the uterus?
promotes coordinated contractions of the pregnant uterus, relaxes the cervix
thus promotes onset of labour
What is Dinopostone?
PGE2 Analog
used as an alternative to oxytocin to induce labour in late stage pregnancy or abortion in early and middle stage pregnancy
delivered by suppository to reduce systemic effects
What is Ritodrine and Salbutamol?
drugs that inhibit uterine contraction
selective beta2-adrenoceptor agonists
inhibit oxytocin induced uterine contraction; cause uterine relaxtion
What is Atosiban?
drug that inhibits uterine contraction
oxytocin receptor antagonist
delays the onset of premature labour
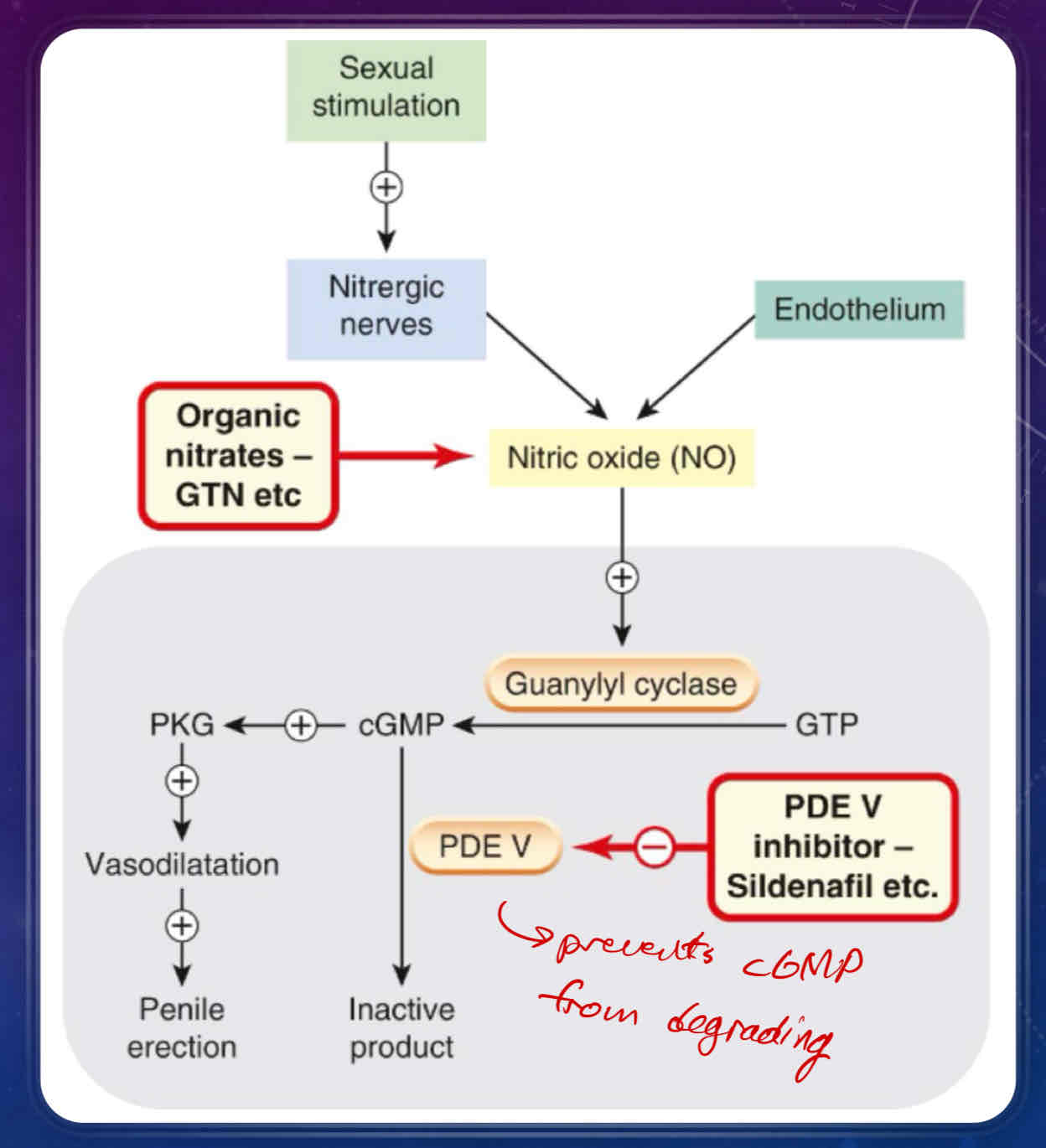
Describe how male erections work
determined by both physiological and psychological factors
vasodilation of arteries and arterioles increase blood flow to the penis allowing engorgement of spongy tissue
compression of penile venules prevents blood from returning from penile tisue, causing erection
innervation is mediated by nitric oxide
What are some physiological causes of erectile dysfunction?
arterial disease
hypogonadism
neuropathies (eg diabetes)
What are some psychological causes of erectile dysfunction?
stress
anxiety
guilt
depression
what are some drugs that can treat psychologically or physiologically caused erectile dysfunction?
anti-psychotics
antidepressants
antihypertensive agents
What is Papaverine and Alprostadil?
used to treat Erectile Dysfunction (ED)
vasodilators injected directly into the penis
can cause a prolonged and painful erection with possible permanent tissue damage
What is Sildenafil and Tadalafil?
used to treat ED
Sildenafil = Viagra
phosphodiesterase type V inhibitors
can be taken orally
enhance erectile response to sexual stimulation (ie does not work independently of sexual desire)
may cause vasodilation in other vascular beds, causing hypotension and headaches
Describe how a penile erection is achieved
sexual stimulation causes nitrergic nerves and the endothelium of the penis to release nitric oxide (NO)
release of nitric oxide activates guanylyl cyclase, which converts GTP to cGMP
cGMP has two fates
conversion/inactivation to inactive product via the PDE V/5 enzyme
Sildenafil (Viagra) is a PDE V inhibitor that promotes penile erection
conversion to PKG, which causes vasodilation in the penis, thus promoting a penile erection