Nuclear Chemistry - Decay reactions and nuclear notation
1/23
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
24 Terms
number at top left of an element?
mass #
number of neutrons and protons added together
number at bottom left of and element?
atomic #
number of protons
knowing this means you know what element it is, and viceversa
number at top right?
charge
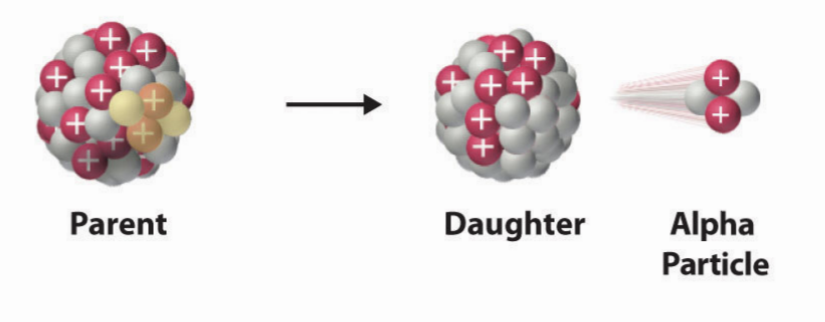
alpha particle
24α
no electrons

from what atomic number does alpha decay start
from Z> 83
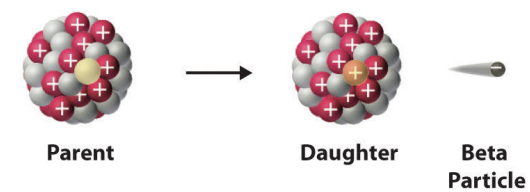
beta particle
electrons originated form the nuclei of atoms in a nuclear decay process
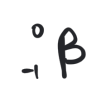
simplest process is the decay of a free neutron
beta particles
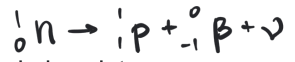
from what atomic number does beta decay happen
nuclei with Z<60 mostly undergo beta emmission
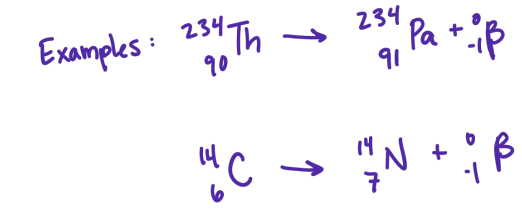
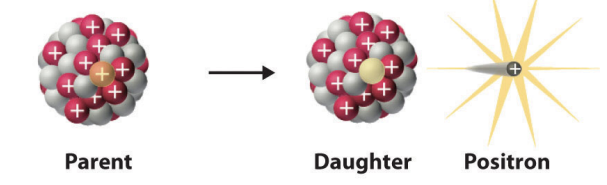
positron
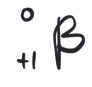
simplest procees is the decay of a free proton
positrons
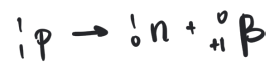
where is positron emission commonly encountered
in artificially produced radioactive nuclei of the lighter elements
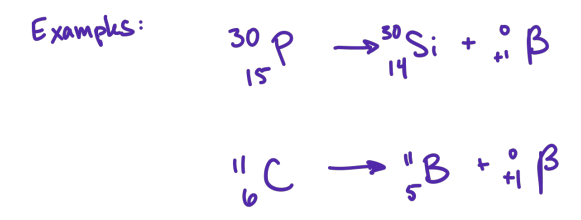
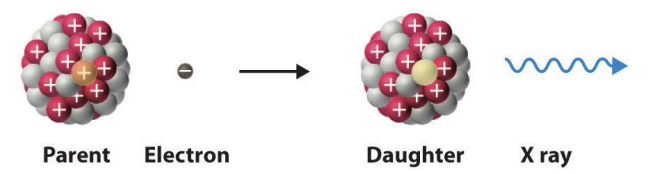
electron captures
achieves the same effect is positron emission through a different process
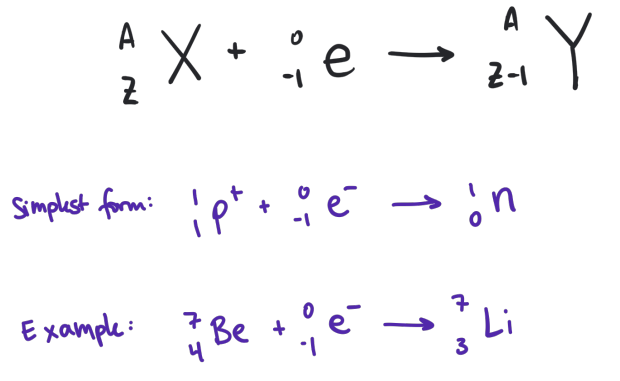
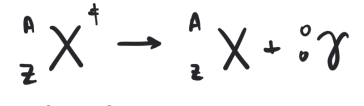
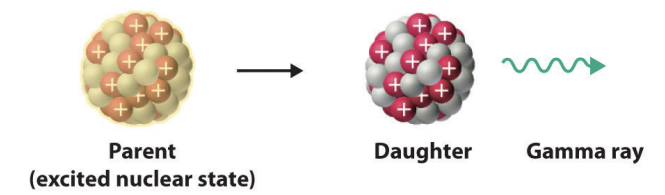
gamma rays
highly penatrating energetic photons
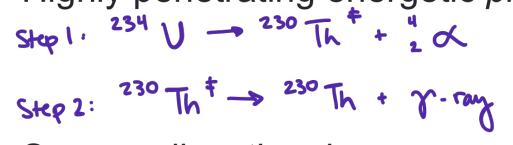
general gamma ray equation
how gamma rays occur
some radiocative decay process that produce alpha or beta particles leave the nucleus in an excited state (engergized)
the nucleus then emits electromagnetic radiation in the form of X-rays or gamma rays, depending on the energy
X-ray a bit weaker(?)
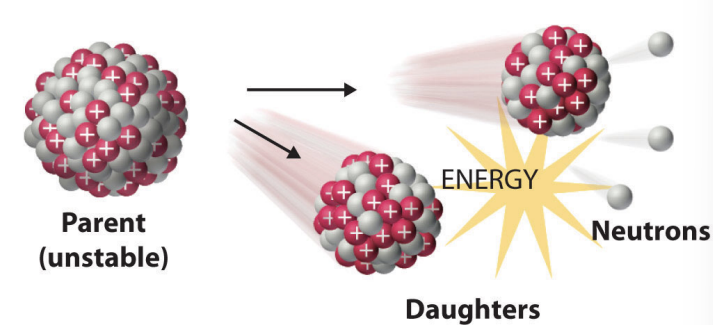
spontaneous fission
happens when the stable nuclei of heavy elements split into two nearly equal daughter nuclei
how can spontaneous fission occur
either naturally or via bombanbardment reactions
often depicted as a chain reaction because neutrons are used and are produced in large quantities
like nuclear bombs
general spontaneous fission reaction
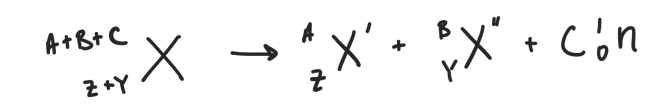
alpha decay model
beta decay model
positron emission model
electron capture model
gamma emission model
s
spontaneous fission model