schrodinger wave equation and the hydrogen atom
1/13
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
14 Terms
del squared
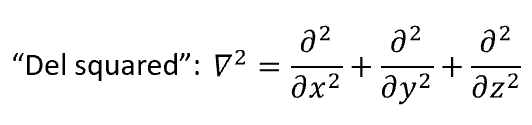
h bar
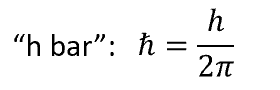
schrodinger wave equation in words and symbols
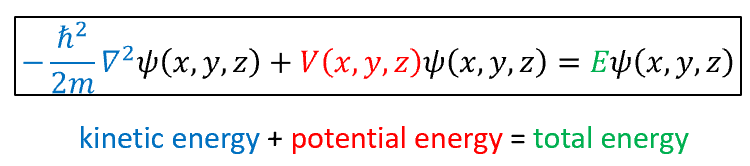
dimensions and time dependency for schrodinger equation
3 dimensional, time-independent
wavefunction symbol and variation
ψ (psi)
varies with position (x, y, z) and contains information on the properties and behaviour of a particle but is not directly measurable
when can the schrodinger equation be solved
only for two-body problems ie nucleus and a single electron (H, He+, Li2+)
born interpretation
ψ2 (referred to as electron density) is proportional to the probability of finding the particle in a small volume dV

heisenberg uncertainty principle
the wave properties of matter mean it is impossible to know precisely the position and momentum of electrons simultaneously - hence quantum mechanics considers the probability of finding electrons within certain volumes.

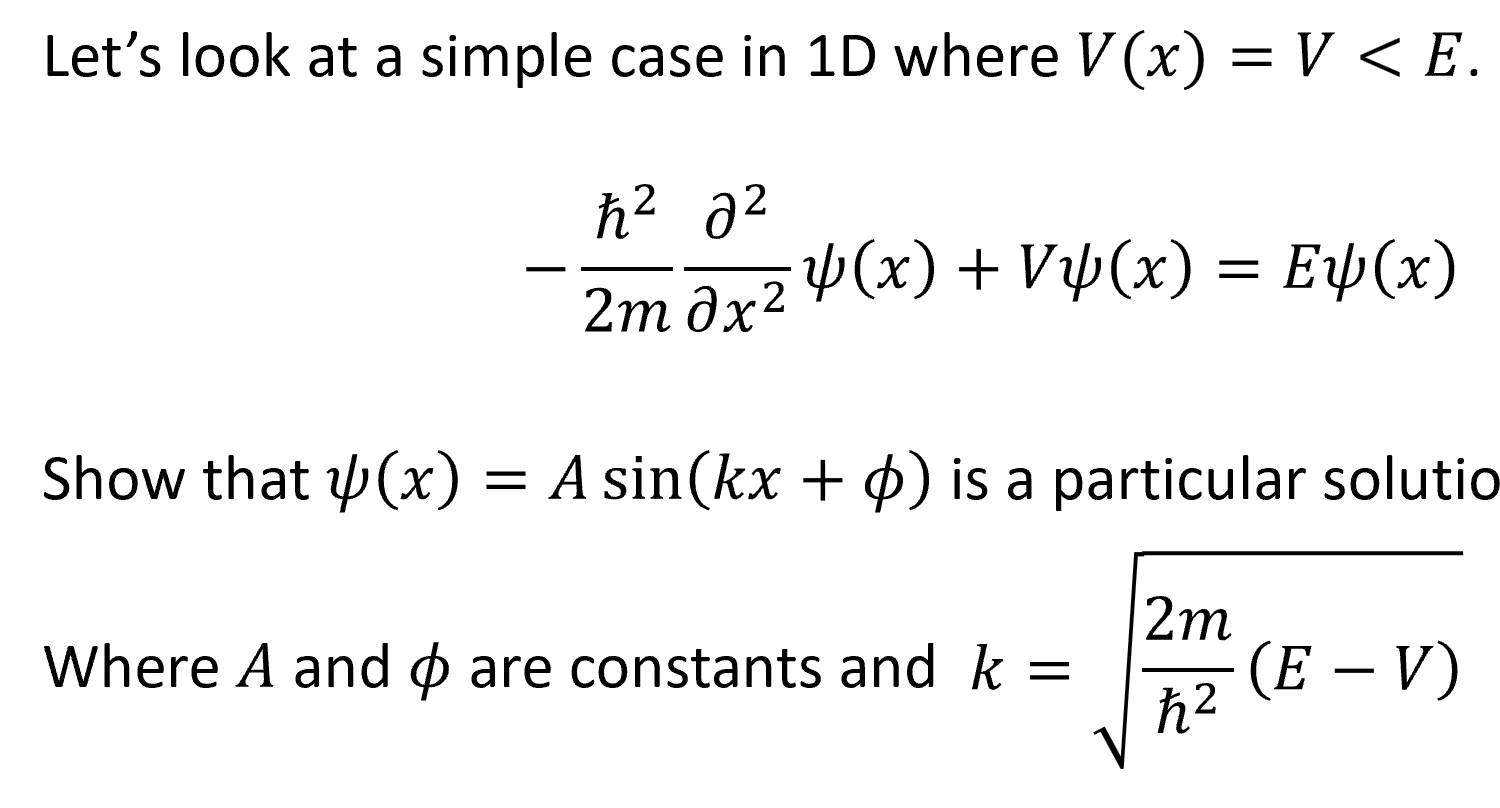
see photos
En for the hydrogen atom from Schrodinger?
matches predictions of Rydberg and Bohr (that it is dependent on principle quantum number n)
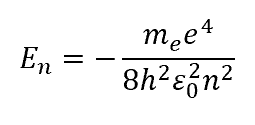
values of n, l and ml from schrodinger

values of l for spdf
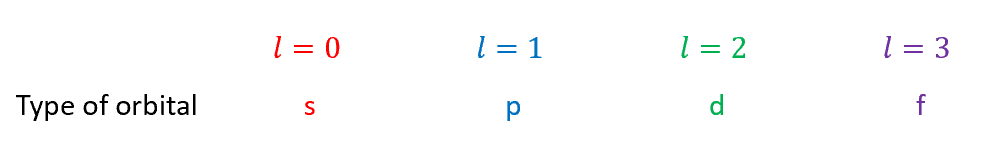
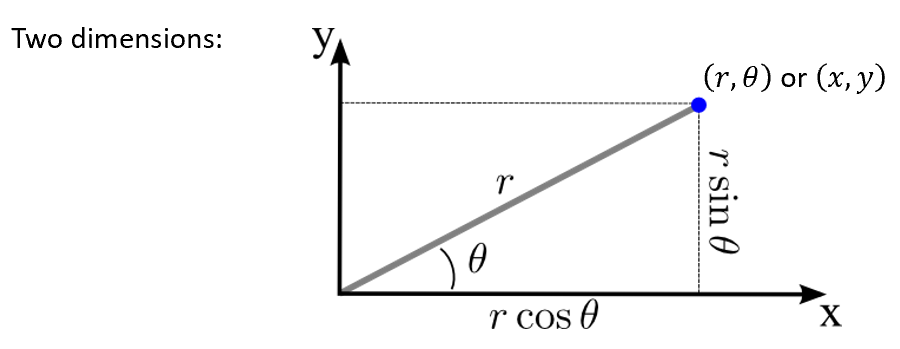
polar coordinates
number of dimensions
diagram
how to convert between cartesian and polar
x = rcosθ
y = rsinθ
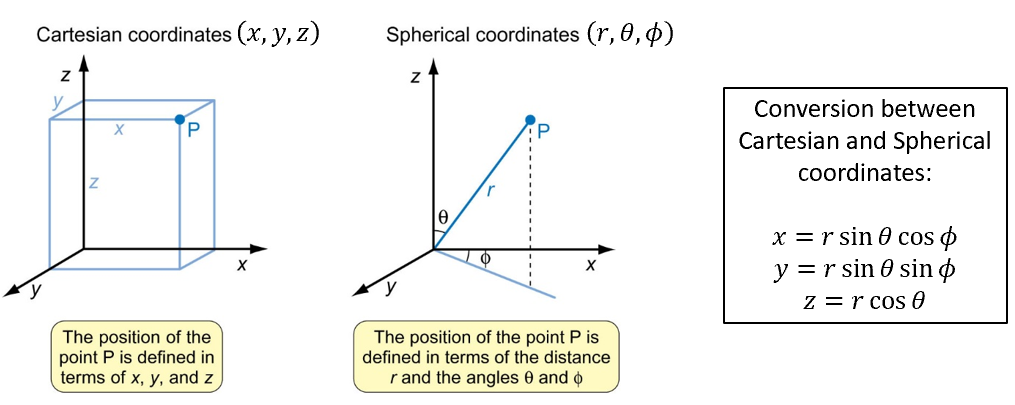
spherical coordinates
why are they called spherical
number of dimensions
diagram
how to convert between cartesian and spherical
atoms are spherical so spherical polar coordinates are used