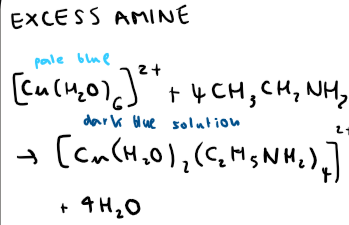Amines
1/20
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
21 Terms
Draw primary amine
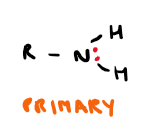
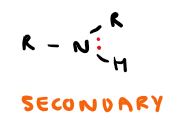
Draw secondary amine
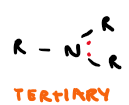
Draw tertiary amine
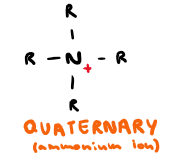
Draw quaternary amine
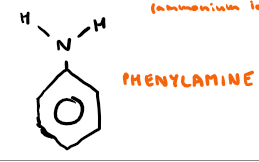
Draw phenylamine
What can amines act as?
Bases
Nucleophiles
How can amines act as nucleophiles?
Due to their lone pair
How can amines act as bases?
Amines have a lone pair of electrons, allowing them to accept protons and act as a base.
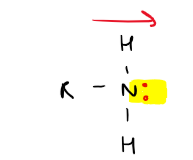
How do protons bond to amine?
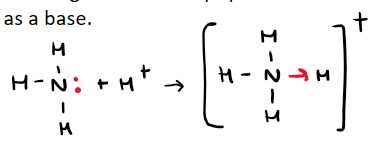
Where do electrons in dative bond originate from?
From lone pair on the nitrogen
What is the trend for basee strength among amines?


Explain this trend for base strength
The higher the electron density -> the greater the availability of the lone pair of electrons on nitrogen -> the stronger the base
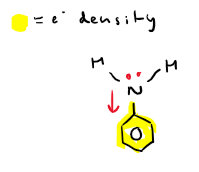
Why do aromatic amines have the lowest base strength?
Benzene pulls lone pair of electrons from nitrogen away into the ring structure. This reduces electron density at nitrogen -> reducing lone pair availability -> making aromatic amines less basic
Why do primary amines have the highest base strength?
Alkyl groups push electrons towards nitrogen -> increasing electron density at nitrogen -> increasing lone pair availability -> making primary amines more basic
What is the reaction between amines and acids? Use HCl and ethylamine as an example + include what product is made
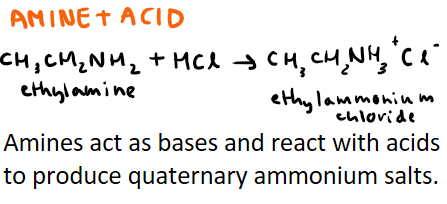
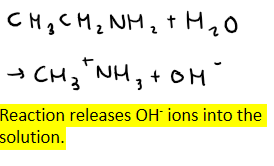
What solutions are formed when amines are dissolved in water?
Alkaline
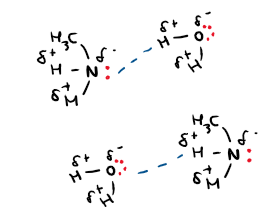
Draw a diagram of amine dissolving in water
The lone pair of electrons on nitrogen can form hydrogen bonds with hydrogen atoms on water molecules. The lone pair on oxygen can also form hydrogen bonds with hydrogen on the amine.
Which amines are soluble and why?

Give the equation for when amine dissociates in water
What is the equation for little ethylamine reacting with hexaaqua copper (II)?
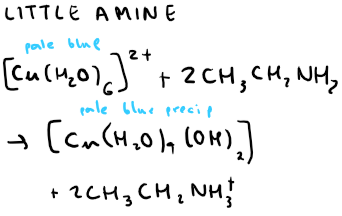
What is the equation for excess ethylamine reacting with hexaaqua copper (II)?