MYCO 3.1B Fungal Energetics & Metabolism
1/102
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
103 Terms
T/F: Fungal energetics focus on how fungi produce energy (i.e., biochemical reactions involved), while fungal metabolism pertains to how fungi synthesize substances essential for their growth & survival
TRUE
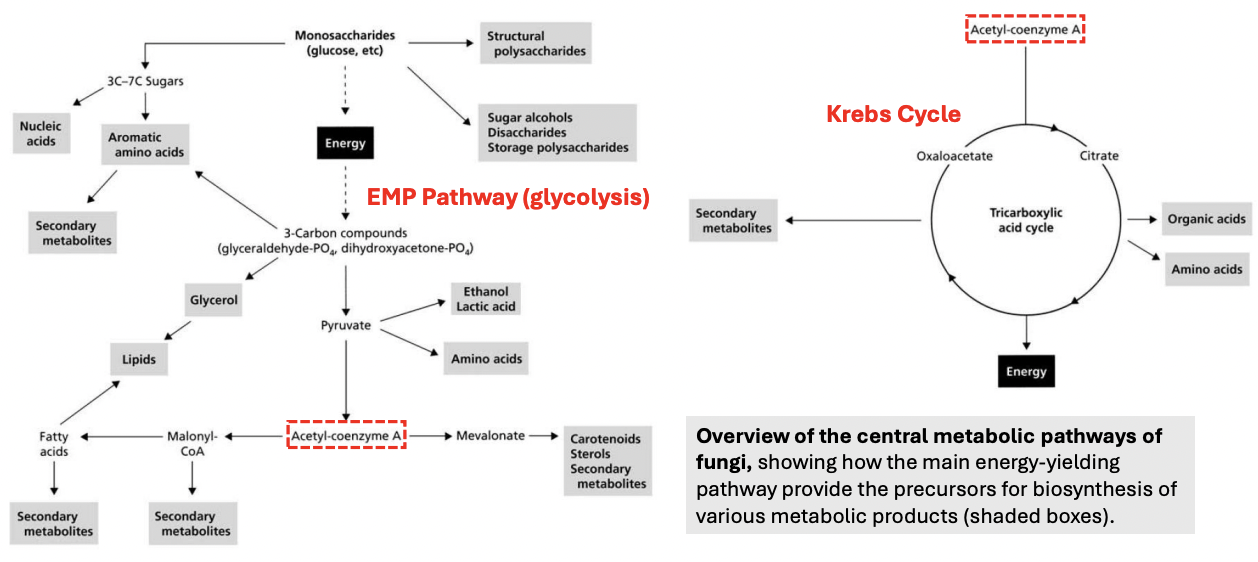
_ are the central reactions in fungal metabolism
Glycolysis (Embden-Myerhoff-Parnas pathway)
Krebs cycle (Citric Acid cycle)
T/F: Aerobic respiration is a process that reduces oxidized organic compounds in a controlled manner
FALSE
Aerobic respiration is a process that oxidizes reduced organic compounds in a controlled manner;
C6H12O6 (reduced) → (oxidized form)
T/F: There are many pathways that can branch out from or go into glycolysis
TRUE
Differentiate substrate-level vs. oxidative phosphorylation
Substrate-level involves direct transfer of phosphate group from a high-energy substrate to ADP, forming ATP
Oxidative uses electron transport chain to generate proton gradient that drives ATP synthesis of ATP synthase
T/F: Both glycolysis and citric acid cycle involves substrate-level phosphorylation, while oxidative phosphorylation is only observed in electron transport chain
TRUE

Oxidizing substances imply _ and thus _
breaking down its bonds and thus releasing energy, prompting creation of reduced temporary energy carriers, e.g., NADH, FADH2
Glycolytic pathway is very fundamental in both _
energy production and biosynthesis
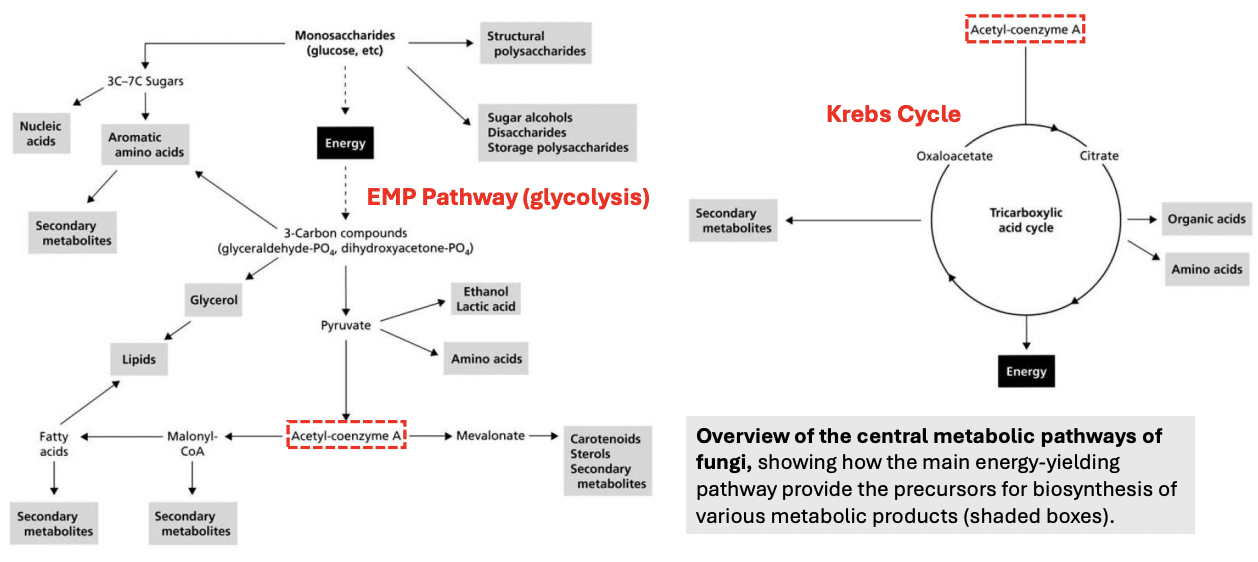
_ releases stored energy in organic molecules for cellular function; hence, a catabolic reaction
Aerobic respiration

_ is the process oxidizing reduced organic compounds in a controlled manner; during this process, free energy is released and stored in a compound called ATP
Aerobic respiration

During respiration, free energy is released and transiently stored in a compound called _
ATP

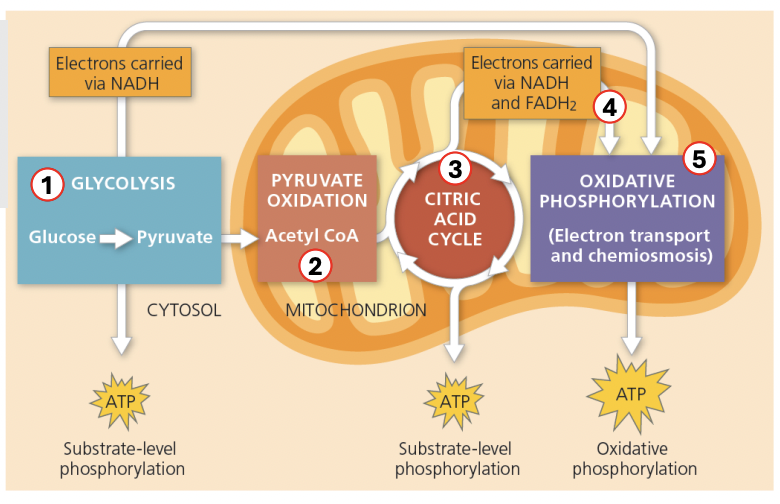
Enumerate 5 pathways involved in aerobic respiration (oxidizes reduced organic molecules to release free energy for cellular function)
Glycolysis = Glucose (6C) → Pyruvate (3C) [cytosol]
Pyruvate decarboxylation / oxidation = Pyruvate (3C) → Acetyl-CoA (2C) [MT matrix]
Citric acid cycle = to generate electron carriers (NADH, FADH2) that will drive ATP synthesis via proton gradient during ETC
Electron transport chain = use movements of electrons across carriers to pump H+ across membrane and generate proton gradient that will drive ATP synthesis via ATP synthase
Oxidative phosphorylation = use electrons to generate ATP
In glycolysis, sucrose (glucose + fructose) may be used to end up with more energy because they produce _
pyruvate + malate
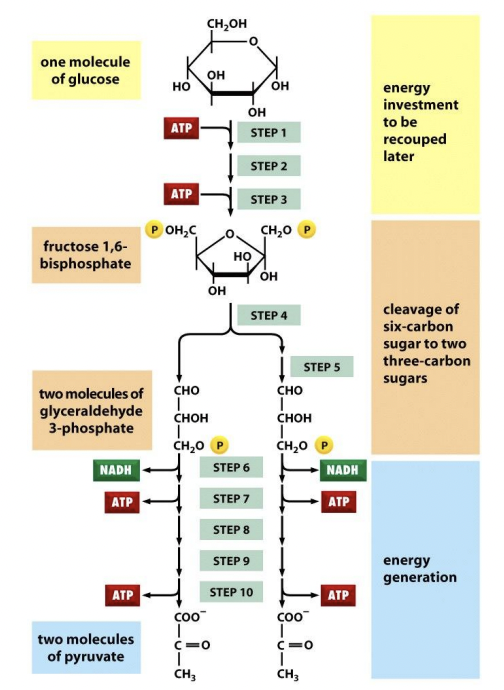
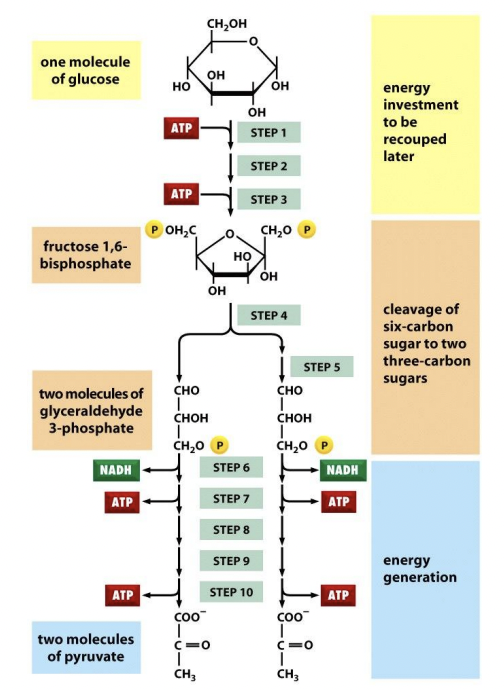
Describe reactants & products of glycolysis (EMP); TLDR glycolysis
Reactants = Glucose (6C) + 2NAD+ + 2 Pi + 2 ADP
Products = 2 pyruvate (3C) + 2 NADH + 2 ATP (net) + 2 H2O + 2 H+ + heat
*4 ATP in total
Glycolysis
(1) Glucose → G6P = - 1 ATP
G6P → F6P
F6P → F-1,6-BP = - 1 ATP
(2) F-1,6-BP → G3P, DHAP
(3) G3P (+Pi) → 1,3-BPG = + 2 NADH
1,3-BPG (-Pi) → 3-PG = + 2 ATP
3-PG → 2-PG
2-PG → PEP = + 2H2O
PEP (-Pi) → Pyruvate = + 2ATP
During _, free energy is released and transiently stored in a compound called ATP
respiration
In glycolysis, glucose may have come from other sources—_ can pass through biochemical pathways (gluconeogenesis) that ultimately make glucose
protein & fat

In glycolysis, _ (glucose + fructose) may be used to end up with more energy because they produce pyruvate + malate
sucrose
Before entering _, the pyruvate from glycolysis must first be decarboxylated to yield _
Krebs cycle (TCA)
Acetyl CoA (2C)

ETC is needed to generate _ needed for chemiosmotic coupling
proton gradient

Explain the process of pyruvate decarboxylation link reaction
Pyruvate is first transported from cytosol → MT matrix via pyruvate translocase
A carboxyl group (1C) is removed from pyruvate (3C) to make 2C derivative; hence the term “decarboxylation”
-COOH is released as CO2
Decarboxylated 2C intermediate is then oxidized, with lost e- picked up by NAD+, forming NADH
Oxidized 2C is now an acetyl group, which will be joined to a Coenzyme A group (derivative from Vit B5) to form Acetyl-CoA

The complete oxidation of Acetyl-CoA reduces _ in a process called the citric acid cycle (TCA)
NAD+ & FAD+ → NADH & FADH2
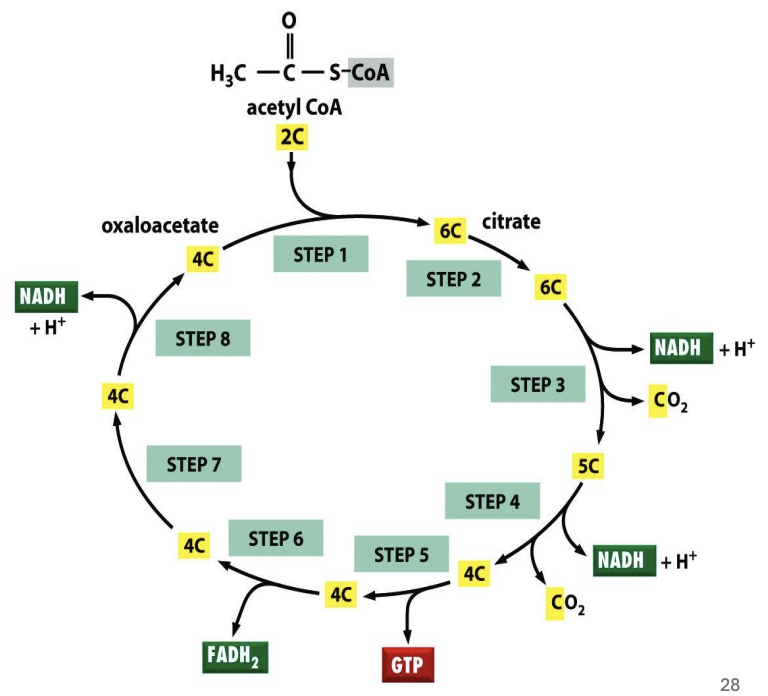
Per completely oxidized Acetyl-CoA, _ are produced
3 NADH
1 FADH2
1 ATP
Explain citric acid cycle (TCA/Krebs)
Acetyl-CoA (2C) condenses with oxaloacetate (OAA, 4C) and undergoes series of conversions that regenerates OAA & produces reduced temporary energy carriers: 3 NADH, 1 FADH2, 1 ATP per completely oxidized Acetyl-CoA
A-CoA + OAA → 6C citrate
6C citrate → 6C isocitrate
6C isocitrate → 5C a-keto
+ CO2
+ NADH
5C a-keto → 4C succinyl CoA
+ CO2
+ NADH
4C succinyl CoA → 4C succinate
+ 1 ATP
4C succinate → 4C fumarate
+ 1 FADH2
4C fumarate → 4C malate
+ NADH
4C malate → 4C OAA
4C OAA + 2C A-CoA → 6C citrate
In the beginning of TCA (Krebs), Acetyl-CoA (2C) is condensed with _ and undergoes series of conversions that regenerates _ but produces reduced temporary energy carriers, including 3 NADH, 1 FADH2, 1 ATP, per completely oxidized Acetyl-CoA
oxaloacetate (OAA)

Overall reaction of aerobic respiration suggests that CO2 is produced as a waste, and this is evident in _ stages
Pyruvate decarboxylation, TCA
_ is needed to generate proton gradient for chemiosmotic coupling
Electron Transport Chain (ETC)

Explain chemiosmotic coupling
*Reduced temporary energy carriers + ATP produced from glycolysis & TCA then participate here
[ETC] Electron transport (transport of electrons from 1 carrier to another) drives the pumps to pump protons across the membrane, allowing protons to establish an electrochemical H+ gradient
NADH is oxidized, releasing electron that goes into ETC that allows H+ to be pumped from matrix to IMS
[Oxidative phosphorylation] Proton gradient is harnessed by ATP synthase to make ATP, as H+ re-enters the MT matrix
![<p><em>*Reduced temporary energy carriers + ATP produced from glycolysis & TCA then participate here</em></p><ol><li><p>[ETC<strong>] Electron transport</strong> (transport of electrons from 1 carrier to another) drives the pumps to pump protons across the membrane, allowing protons to <u>establish an electrochemical H+ gradient</u></p><ol><li><p><em>NADH is oxidized, releasing electron that goes into ETC that allows H+ to be pumped from matrix to IMS</em></p></li></ol></li><li><p>[Oxidative phosphorylation] Proton gradient is <strong>harnessed by ATP synthase to make ATP, </strong>as <u>H+ re-enters the MT matrix</u></p></li></ol><p></p>](https://knowt-user-attachments.s3.amazonaws.com/d8a53284-d19c-4179-8b3c-1179f91d4282.png)
Cells use _ to mill energy from an electron donated by either NADH or FADH2 to move H+ from the MT matrix into intermembrane space (IMS)
electron transport chain

Hydrogen cannot enter MT matrix except through _
ATP synthase
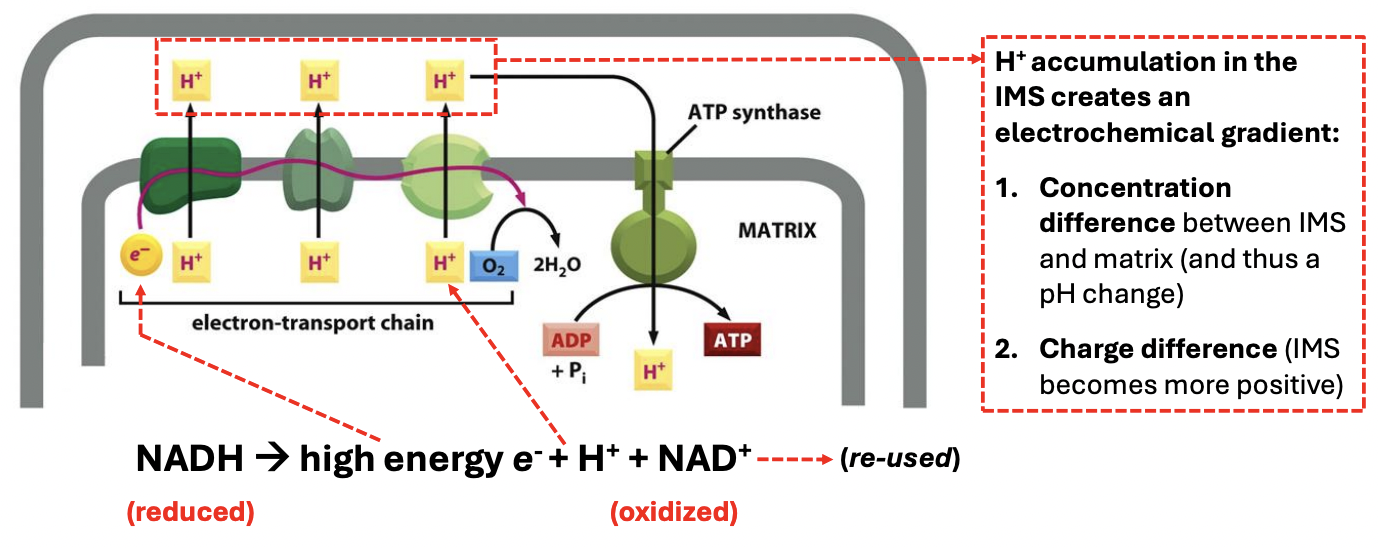
H+ accumulation in IMS creates an electrochemical gradient due to 2 things, including _
cocha
H+ concentration difference in IMS & matrix, thus a pH change (lower pH in IMS than matrix)
Charge difference = IMS is more positive
Explain 1st step of chemiosmotic coupling: ETC
NADH+ (reduced) → (oxidized) high energy e- + H+ + NAD+ (re-used)
High energy e- travel thru ETC complexes, driving active pumping of H+ from matrix into IMS
This results in H+ accumulation in IMS that creates an electrochemical gradient
Concentration difference → change in pH (low outside)
Charge difference → IMS more electropositive
H+ goes back into MT matrix via ATP synthase (passive transport)
NAD⁺ for reuse in glycolysis and the citric acid cycle

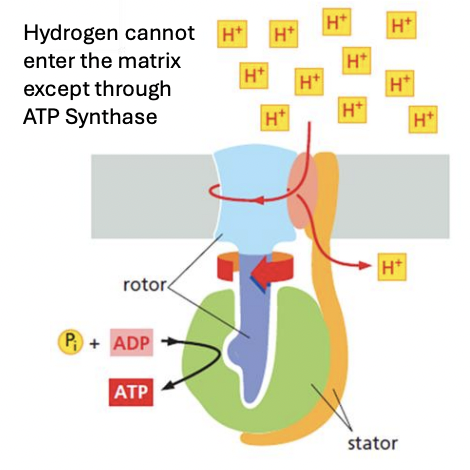
_ functions via rotational catalysis through the binding change mechanism that drives ATP synthesis
ATP synthase
ATP synthase functions via _ through _ that drives ATP synthesis
Rotational catalysis
Binding change mechanism (cycling between loose ADP, tight ATP, open or empty conformation)
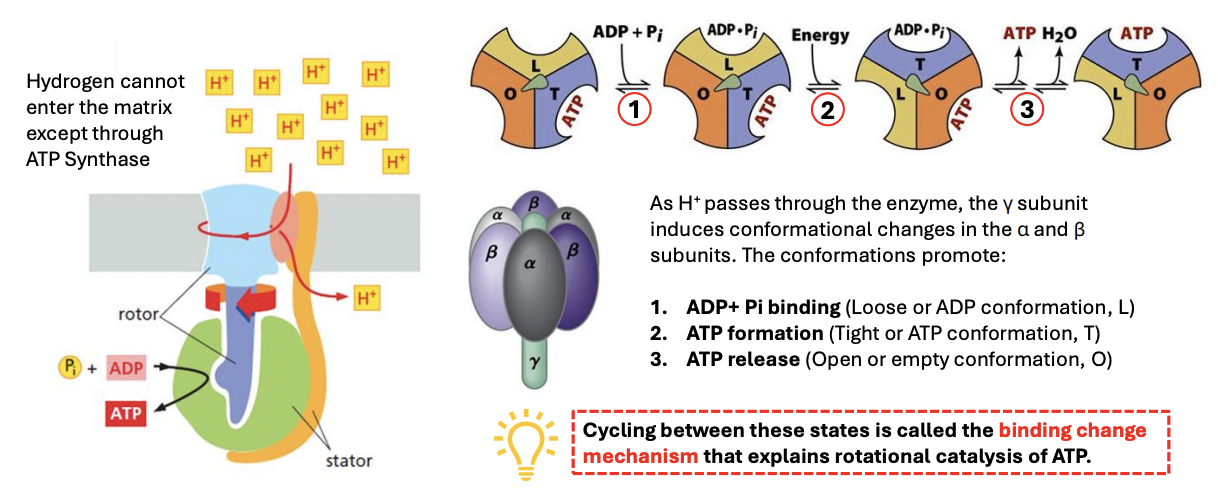
T/F: Hydrogen cannot enter the MT matrix except through ATP synthase
TRUE
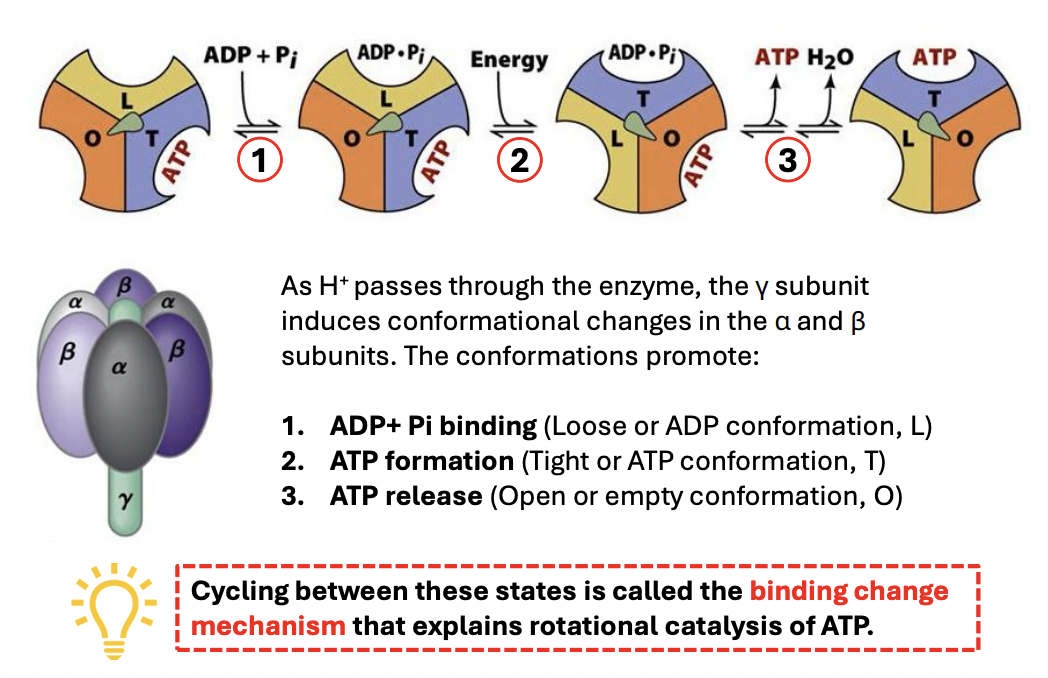
T/F: F1 of ATP synthase has 3 chemically similar but conformationally distinct alpha, beta protomers—each of which has 1 catalytic site for protein synthesis (L,T,O)
FALSE
F1 of ATP synthase has 3 chemically identical but conformationally distinct alpha, beta protomers—each of which has 1 catalytic site for protein synthesis (L,T,O)
Each protomer (a or b) (within rotor) has 1 catalytic site, either _
Loose conformation = binds ligands loosely
Tight conformation = binds ligands tightly, catalytically active
Open / empty conformation = has very low affinity for substrates/products
Explain binding change mechanism that explains rotational catalysis of ATP
H+ accumulates in IMS due to ETC pumping H+ out into IMS
H+ cannot enter MT matrix except through ATP synthase
ATP synthase functions via rotational catalysis through binding change mechanism (cycling between L,T,O states) that drive ATP synthesis, such that
As H+ passes through ATP synthase, gamma subunit induces conformational changes in alpha & beta subunits, promoting the ff:
Initially = ATP in T conformation, then ADP+Pi binding in L conformation
Addition of energy causes L site to become T, O to L, T to O
This causes ADP+PI in T, ATP in O, causing its release, with H2O as byproduct of hydrolysis
New ATP then spontaneously forms in the new T site
After 2 such sequences, enzyme returns to its initial state

T/F: ATP synthase directly uses the energy of proton flow to synthesize ATP, rather than to induce conformational changes that drive ATP synthesis
FALSE
Proton flow induces conformational changes that allow ATP synthesis, rather than directly providing energy to form the bond
T/F: The release of ATP from ATP synthase requires energy input from the proton gradient
TRUE
The energy from proton flow is required to induce the conformational change (from T to O) that allows ATP release
T/F: ATP is synthesized in the Loose (L) conformation of ATP synthase
FALSE
ATP is synthesized in the Tight (T) conformation of ATP synthase
T/F: A single full rotation of the γ subunit of ATP synthase results in the synthesis and release of three ATP molecules
TRUE
The γ subunit rotates in 120° steps, and a full 360° rotation cycles through three catalytic sites, each producing one ATP
*120deg = 1 ATP in 1 site
Full rotatio 360 deg = 1 ATP in each of 3 sites = 3 ATP
The complete oxidation of 1 glucose molecules produces an equivalent of _
36 - 38 ATP

1 NADH forms _ ATP, while 1 FADH2 forms _ ATP
NADH = 3 ATP
FADH2 = 2 ATP
Explain energy generation in aerobic respiration

T/F: The proximity of ADP + Pi is what causes production of ATP
TRUE
The general conversion of ATP produced by NADH = 3, FADH = 2, but in reality, it is actually close to _
NADH = 2.5/molecule, FADH2 = 1.5/molecule
T/F: Fungi can perform fermentations when O2 is not available as electron donor
FALSE
Fungi can perform fermentations when O2 is not available as electron acceptor
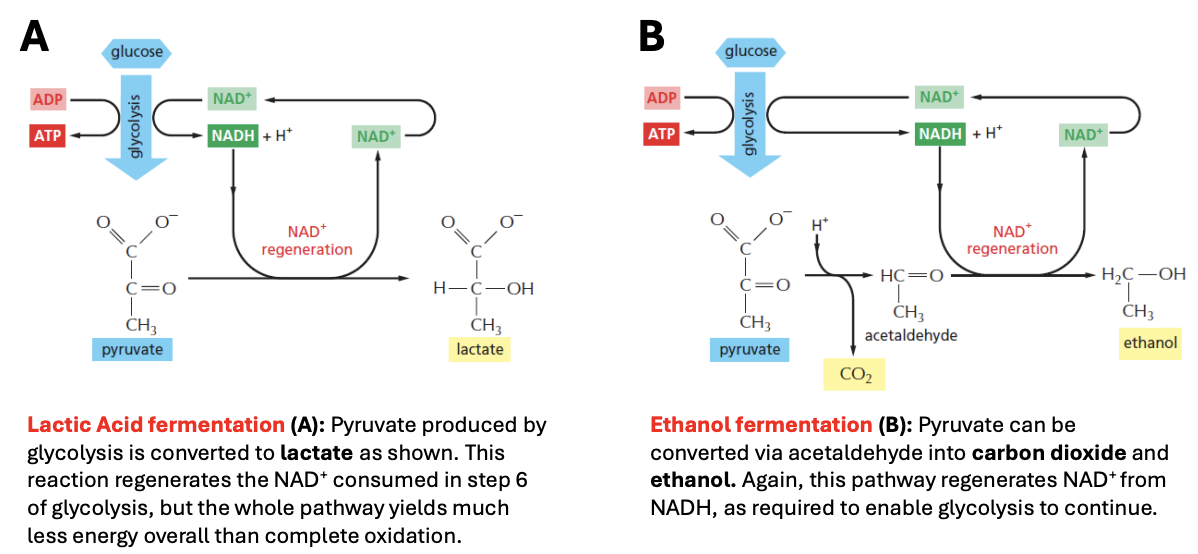
Explain lactic acid & ethanol fermentation pathways
Lactic acid fermentation
Pyruvate from glycolysis is directly converted to (reduced to) lactate, using NADH and thus oxidizing this back to NAD+ (to be used for another round of glycolysis)
The whole pathway yields much less overall energy than complete oxidation
Ethanol fermentation
Pyruvate is converted to (oxidized to) acetaldehyde, releasing CO2
Acetaldehyde is reduced to ethanol by oxidizing NADH in the process, thus regenerating NAD+ necessary for glycosis to continue
Goal: to regenerate NAD+ so glycosis can continue to produce ATP under anaerobic conditions

T/F: Ethanol fermentation produces more ATP per glucose molecule than lactic acid fermentation
FALSE
Both only produce 2 ATP per glucose via glycolysis
T/F: In ethanol fermentation, NADH is oxidized to NAD⁺ during the conversion of pyruvate to acetaldehyde
FALSE
In ethanol fermentation, NADH is oxidized to NAD⁺ during the conversion/reduction of acetaldehyde to ethanol
T/F: Only ethanol fermentation releases CO2, making it the only fermentation pathway that contributes to bread rising
TRUE
Lactic acid fermentation does not release CO₂, whereas ethanol fermentation does—this is why yeast makes bread rise
T/F: The production of ethanol and CO2 in yeast fermentation is necessary for regenerating ATP
FALSE
The fermentation process itself does not generate ATP; ATP is only produced during glycolysis
T/F: Lactic acid fermentation occurs in fungi and some bacteria, but never in human cells
FALSE
human muscle cells undergo lactic acid fermentation during anaerobic conditions like intense exercise
T/F: Fungi evolved to do fermentation despite yielding more ATP via aerobic respiration to survive under anaerobic conditions
TRUE
_ is the starting molecule for Pentose Phosphate Pathway
G6P
Explain Pentose Phosphate Pathway
*Functions for both energy production & biosynthesis
Under oxidative phase
G6P → 6-phosphogluconate
NADP+ → NADPH, which is then used to regenerate glutathione back to its active form to neutralize ROS, e.g., H2O2
NADPH → 2 GSH to GSSG, which converts H2O2 → H2O
6-phosphoglucanate → Ribulose-5-phosphate, producing another NADPH, releasing CO2
Ribu-5-P → Ribose-5-phosphate, producing sugar backbones for synthesis of nucleotides, coenzymes, DNA, RNA
Under nonoxidative phase
Ribulose-5-P can be converted back to G3P, F6P, which can reenter EMP and produce other types of sugars to be used, for instance, for cell wall synthesis
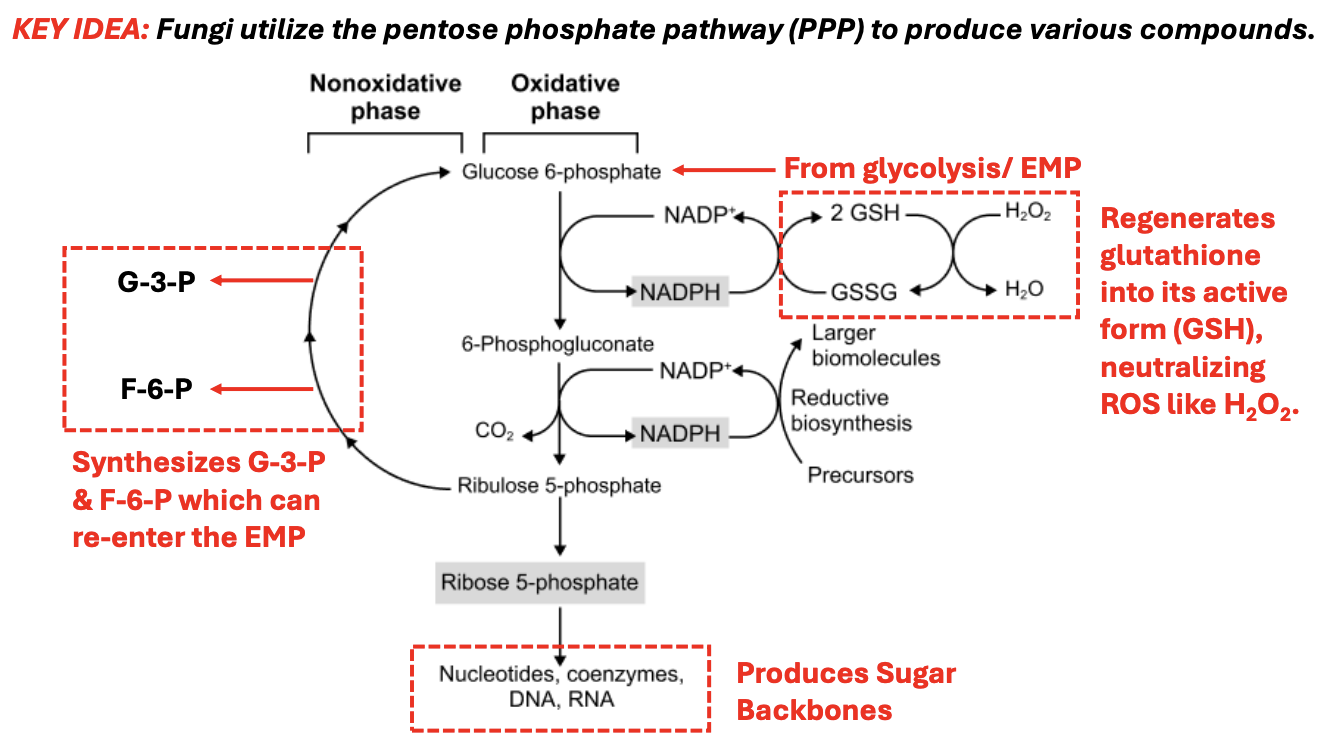
T/F: Under nonoxidative phase of PPP, ribose-5-phosphate can be converted to G3P or F6P to re-enter EMP
FALSE
Under nonoxidative phase of PPP, ribulose-5-phosphate can be converted to G3P or F6P to re-enter EMP for production of other sugar types
T/F: In PPP, ribulose-5-phosphate can be used as sugar backbones for producing nucleotides, coenzymes, DNA, RNA
FALSE
In PPP, ribose-5-phosphate can be used as sugar backbones for producing nucleotides, coenzymes, DNA, RNA
T/F: In Pentose Phosphate Pathway, oxidative phase is irreversible, nonoxidative is reversible
TRUE
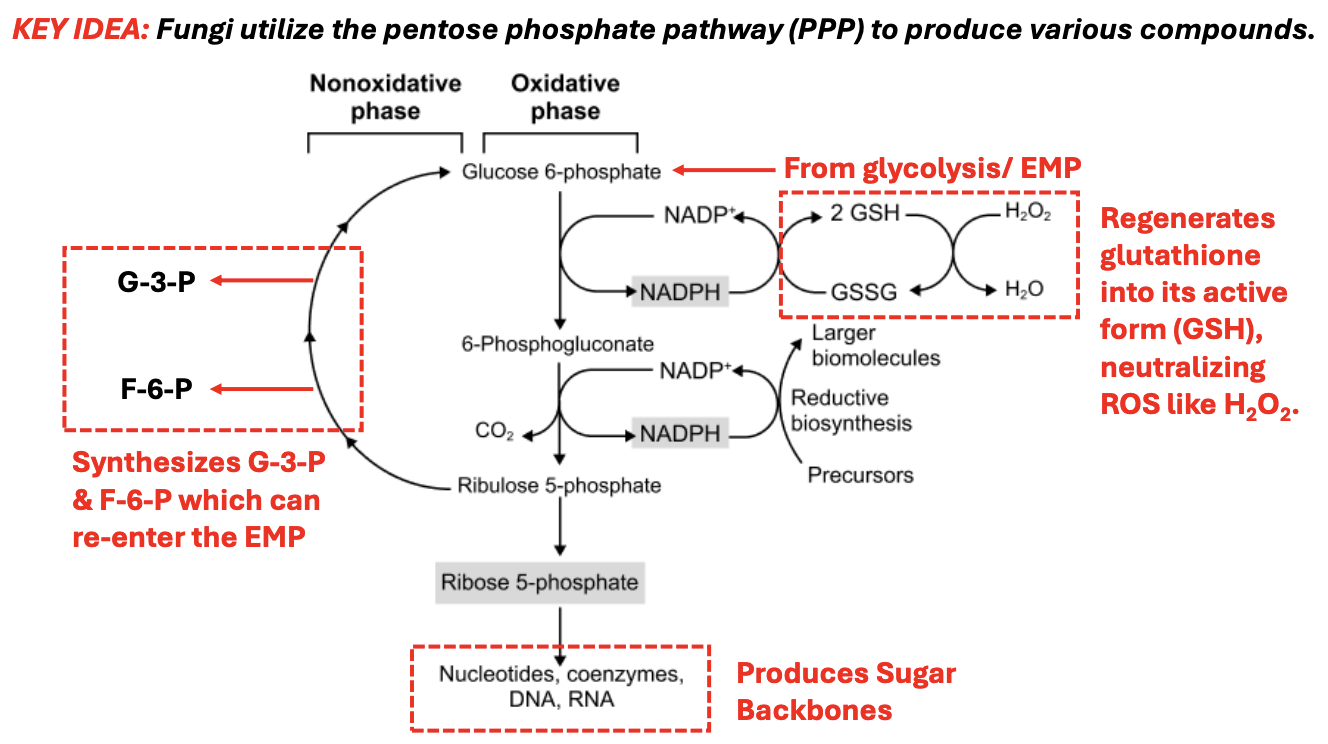
T/F: In Pentose Phosphate Pathway, the nonoxidative phase does not regenerate NADPH but allows sugar rearrangements
TRUE
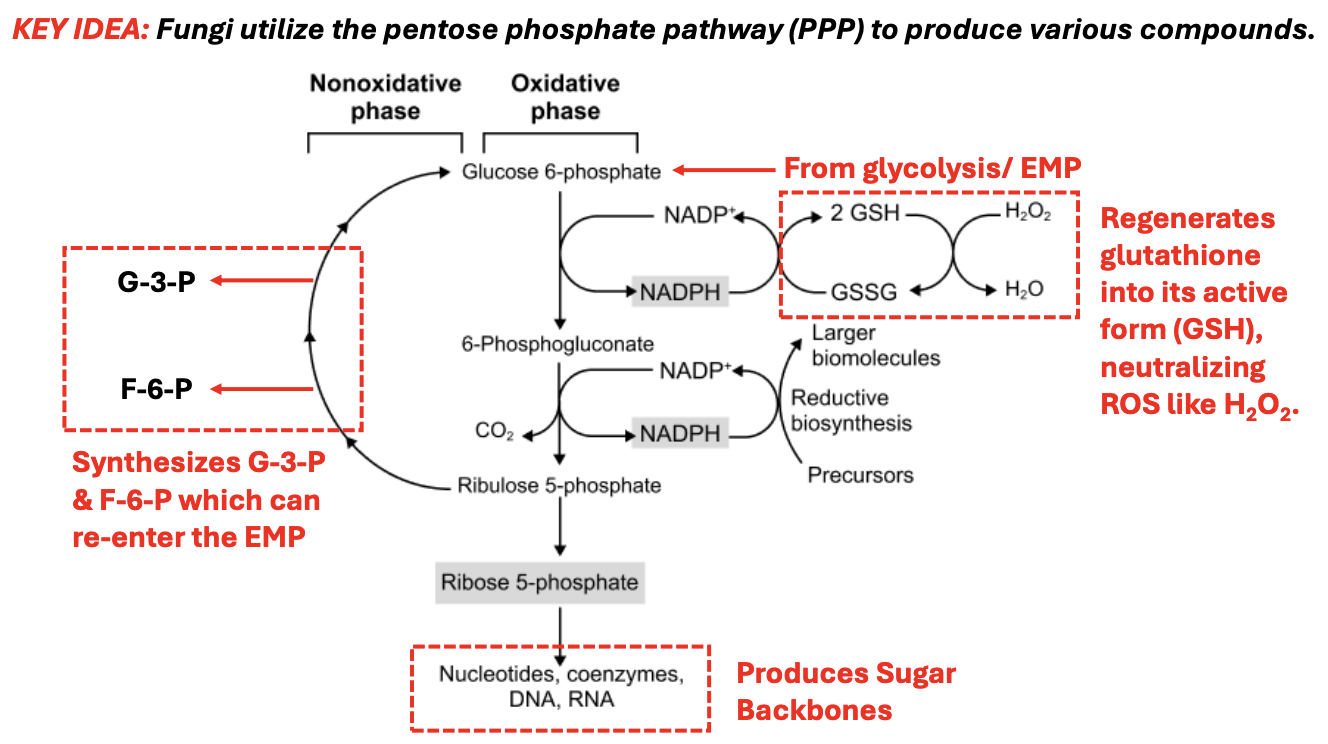
In PPP, _ produced from converting G6P to 6-phosphogluconate reduces glutathione (GSSG) back to its active form (GSH)
NADPH

_ detoxifies ROS like H2O2 by converting it to H2O, preventing oxidative damage
Glutathione (GSH)
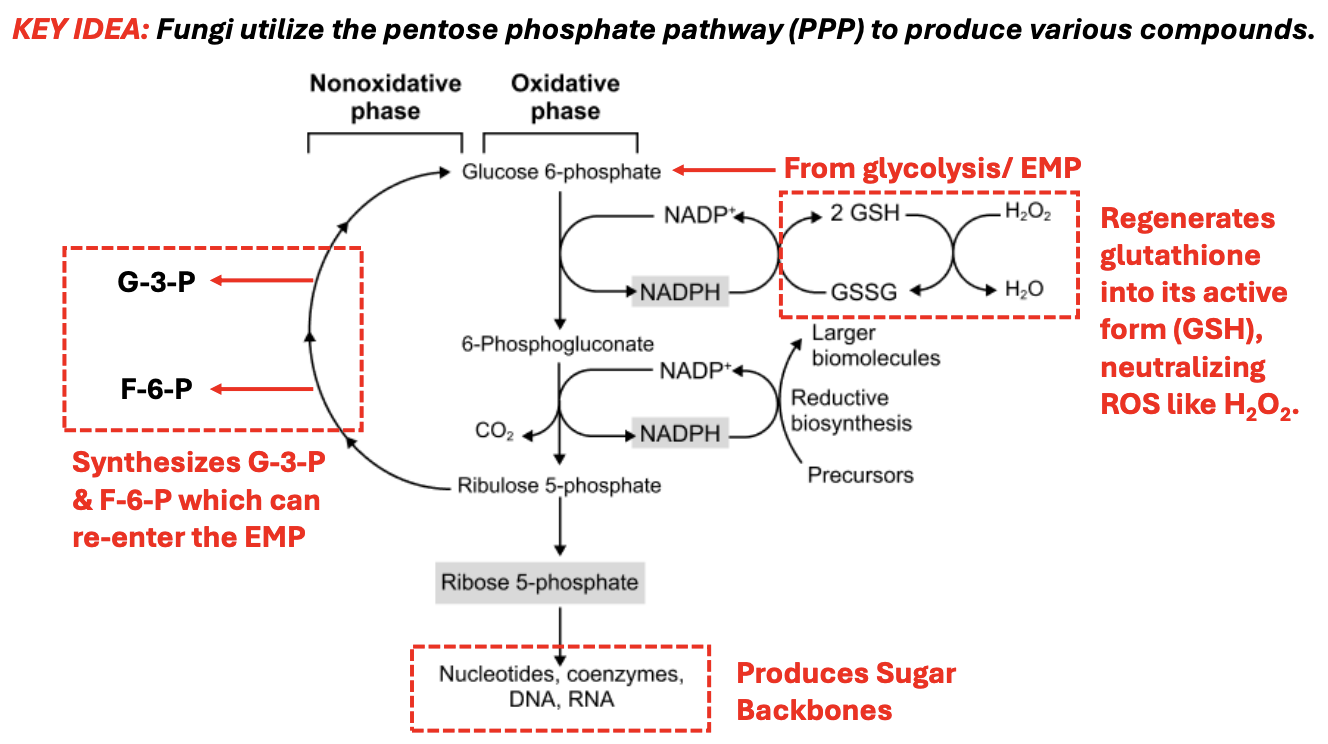
T/F: NADPH generated from the PPP is essential for neutralizing reactive oxygen species (ROS) by maintaining glutathione in its reduced form (GSH)
TRUE
T/F: A deficiency in the PPP would have the greatest impact on cells that experience high oxidative stress, such as red blood cells
TRUE
Red blood cells lack mitochondria and depend on NADPH from the PPP to regenerate glutathione and prevent oxidative damage
T/F: Inhibition of glucose-6-phosphate dehydrogenase (G6PD), the first enzyme in the PPP, would impair a cell’s ability to synthesize nucleotides and manage oxidative stress
TRUE
G6PD deficiency reduces NADPH levels, making cells vulnerable to oxidative stress and limiting their ability to produce ribose-5-phosphate for nucleotide synthesis
T/F: In PPP, ribulose-5-phosphate can regenerate G6P under oxidative conditions but indirectly via glycolytic intermediates, e.g., G3P, F6P
FALSE
In PPP, ribulose-5-phosphate can regenerate G6P under nonoxidative conditions but indirectly via glycolytic intermediates, e.g., G3P, F6P
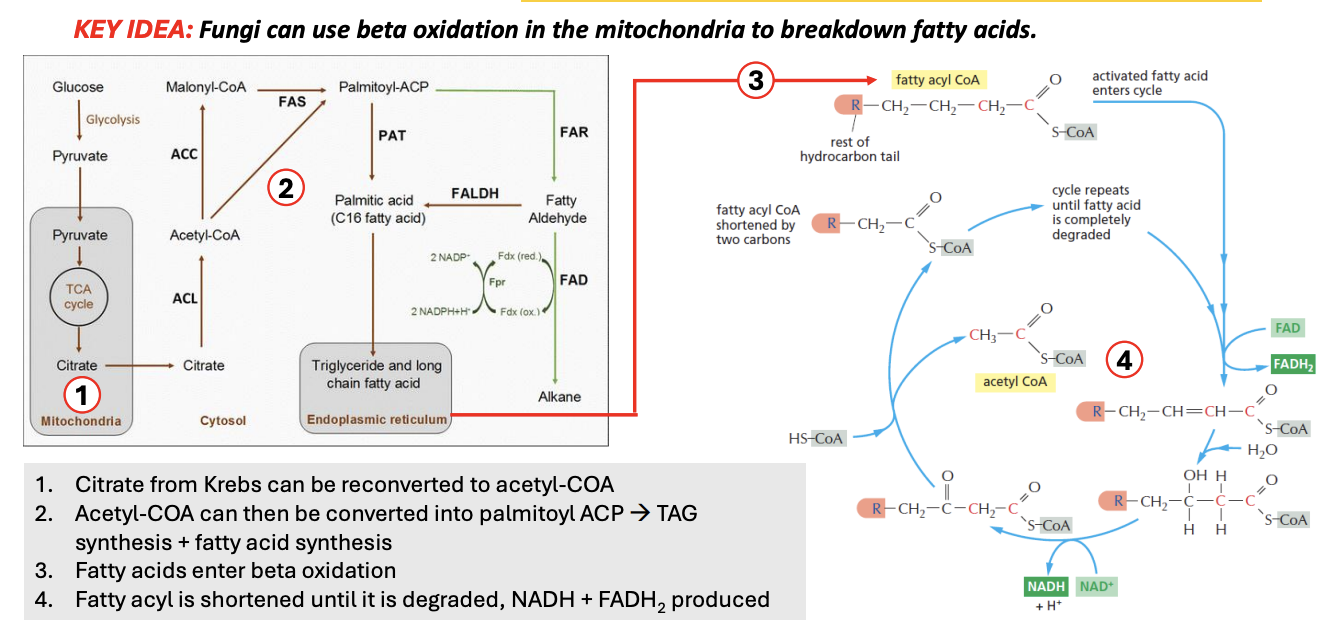
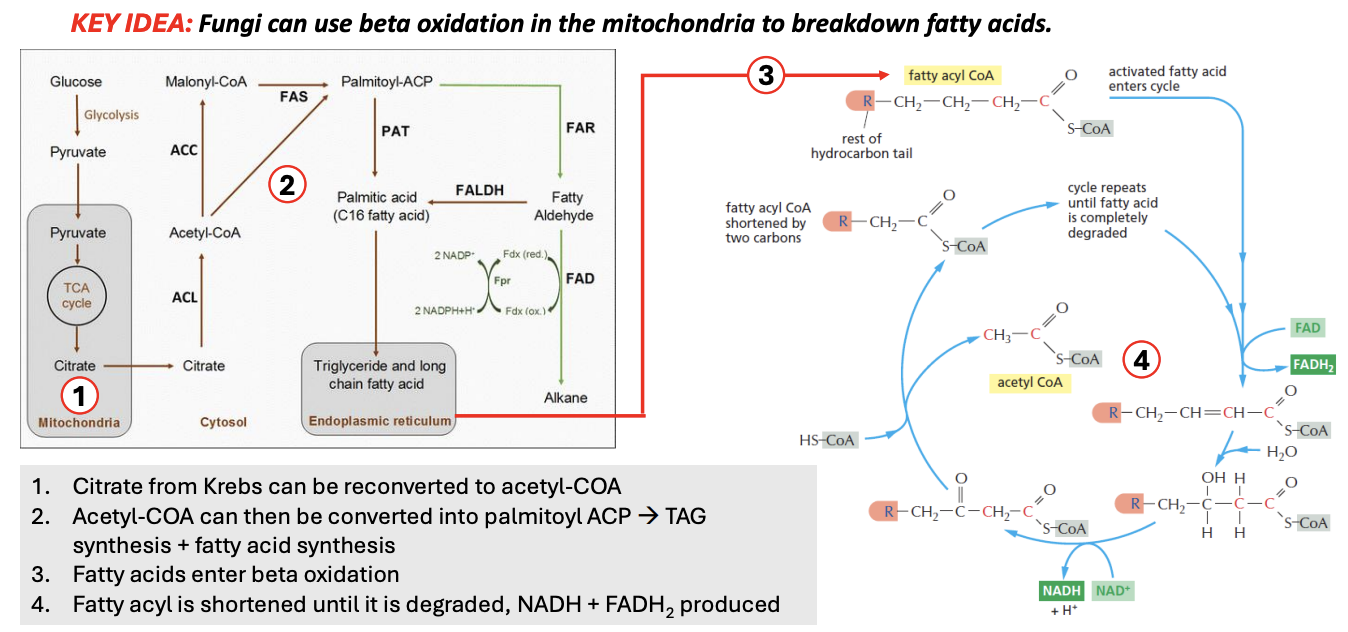
_ refers to the metabolic process where fatty acids are broken down in the mitochondria into acetyl-CoA, NADH, FADH2, which can then enter TCA and ETC to produce ATP
Beta-oxidation
T/F: Both lactic acid and ethanol fermentation occur in cytosol
TRUE
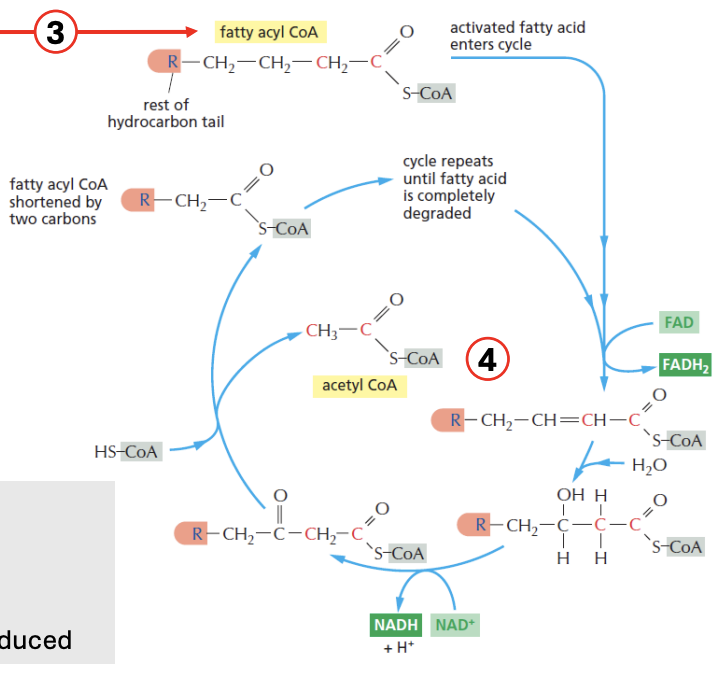
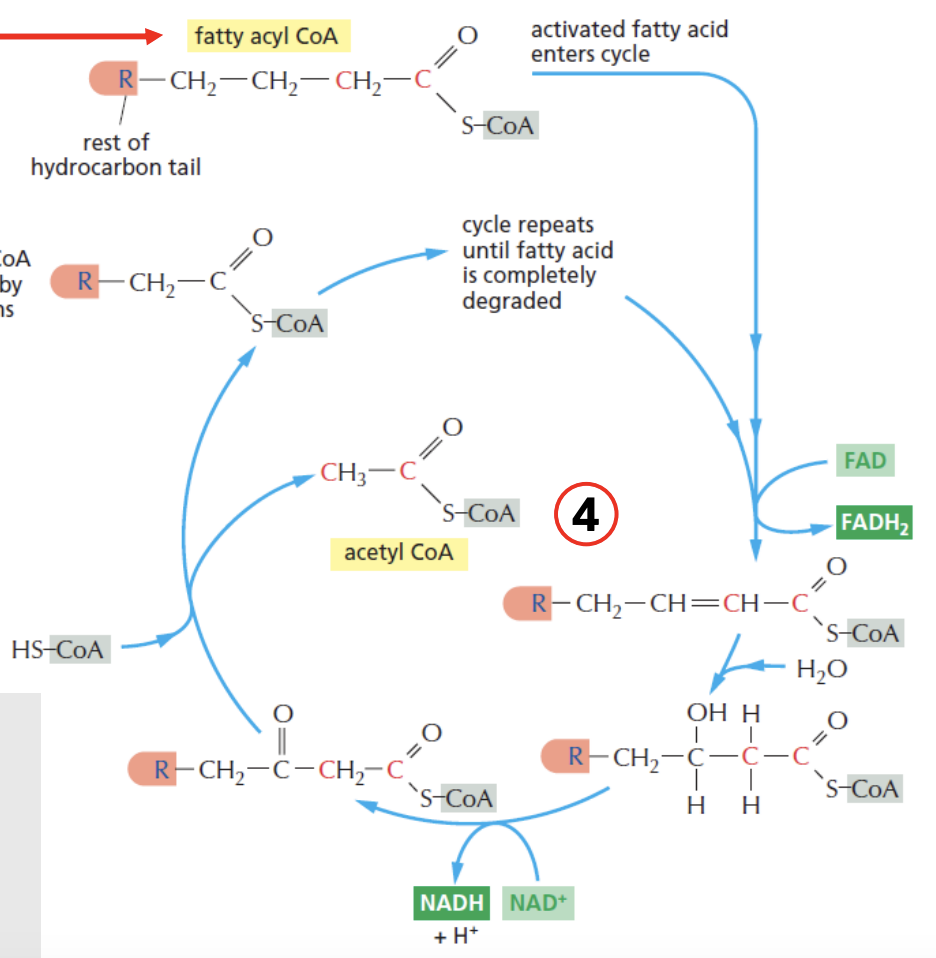
The fatty acyl CoA is shortened by _ carbons per 1 beta oxidation cycle
2
Beta oxidation cycle of fatty acids continue until _
fatty acid is completely degraded
Explain beta-oxidation of fatty acids
[cytosol] Glu → Pyruvate (glycolysis)
Pyru → [mito] TCA → citrate
Citrate is pumped out to cytosol
Citrate → acetyl-CoA → Palmitoyl-ACP (precursor for first FA)
→ Palmitic acid (C16 FA), which then enters ER, where no. of carbons can be changed (shortened/lengthened), producing triglycerides + long chain fatty acids
TAG + LCFA enter mitochondria, undergoing beta-oxidation cycle
Per beta-oxi cycle, fatty acyl CoA is shortened by 2 carbons
e.g., 4C fatty acyl CoA, after producing 1 acetyl CoA (which is 2 carbon), reenters beta-oxi cycle as 2C fatty acyl CoA
NADH, FADH2 is produced in the process
Cycle repeats until the entire fatty acyl CoA is completely degraded
![<ul><li><p>[cytosol] Glu → Pyruvate (glycolysis)</p></li><li><p>Pyru → [mito] TCA → citrate</p><ul><li><p><strong>Citrate</strong> is pumped out to cytosol</p></li><li><p>Citrate → <strong>acetyl-CoA → Palmitoyl-ACP</strong> (precursor for first FA)</p></li><li><p>→ <strong>Palmitic acid (C16 FA),</strong> which then enters <u>ER</u>, where <u>no. of carbons can be changed</u> (shortened/lengthened), producing<strong> triglycerides + long chain fatty acids</strong></p></li></ul></li><li><p>TAG + LCFA enter <u>mitochondria</u>, undergoing beta-oxidation cycle</p><ul><li><p>Per beta-oxi cycle,<strong> fatty acyl CoA is shortened by 2 carbons</strong></p></li><li><p>e.g., 4C fatty acyl CoA, after producing 1 acetyl CoA (which is 2 carbon), reenters beta-oxi cycle as 2C fatty acyl CoA</p><ul><li><p><em>NADH, FADH2 is produced in the process</em></p></li></ul></li><li><p><em><u>Cycle repeats until the entire fatty acyl CoA is completely degraded</u></em></p></li></ul></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/73f4be32-28cd-4e78-abef-055006da7366.png)
Triglycerides and long-chain FAs are sent to _ for beta-oxidation
mitochondria
Fatty acids are first activated to _ in the mitochondria by acyl-CoA synthetase before entering beta-oxidation
fatty acyl-CoA
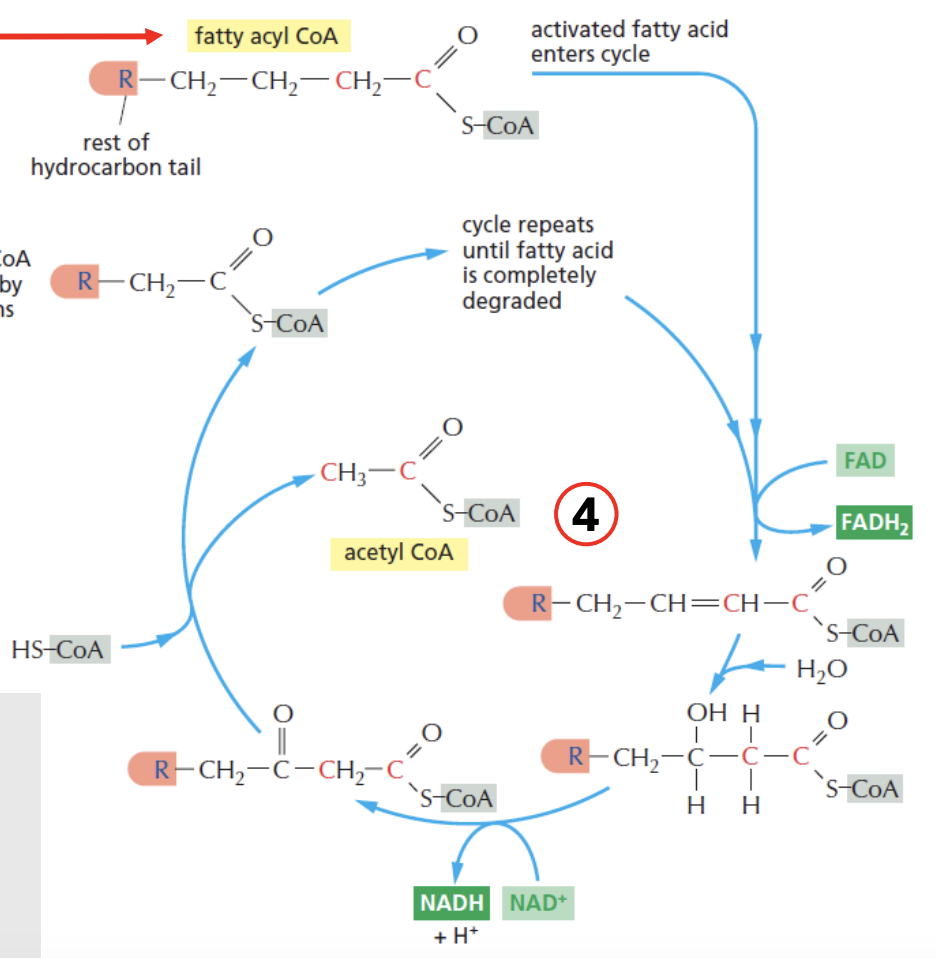
During beta-oxidation of fatty acids, _ are the energy carriers produced
NADH + FADH2
T/F: Each round of β-oxidation shortens a fatty acid chain by one carbon, producing Acetyl-CoA
FALSE
Each round of β-oxidation shortens a fatty acid chain by two carbon, producing Acetyl-CoA

T/F: Fungal β-oxidation is essential for generating Acetyl-CoA, which can enter both the TCA cycle and gluconeogenesis
TRUE
T/F: The first step of β-oxidation involves the activation of fatty acids in the mitochondria using ATP
TRUE
Fatty acids are activated to fatty acyl-CoA by acyl-CoA synthetase before entering β-oxidation
T/F: Fatty acid synthesis and β-oxidation occur in the same cellular compartment to ensure efficient regulation
FALSE
FA synthesis = cytosol + ER
Beta-oxidation = mitochondria
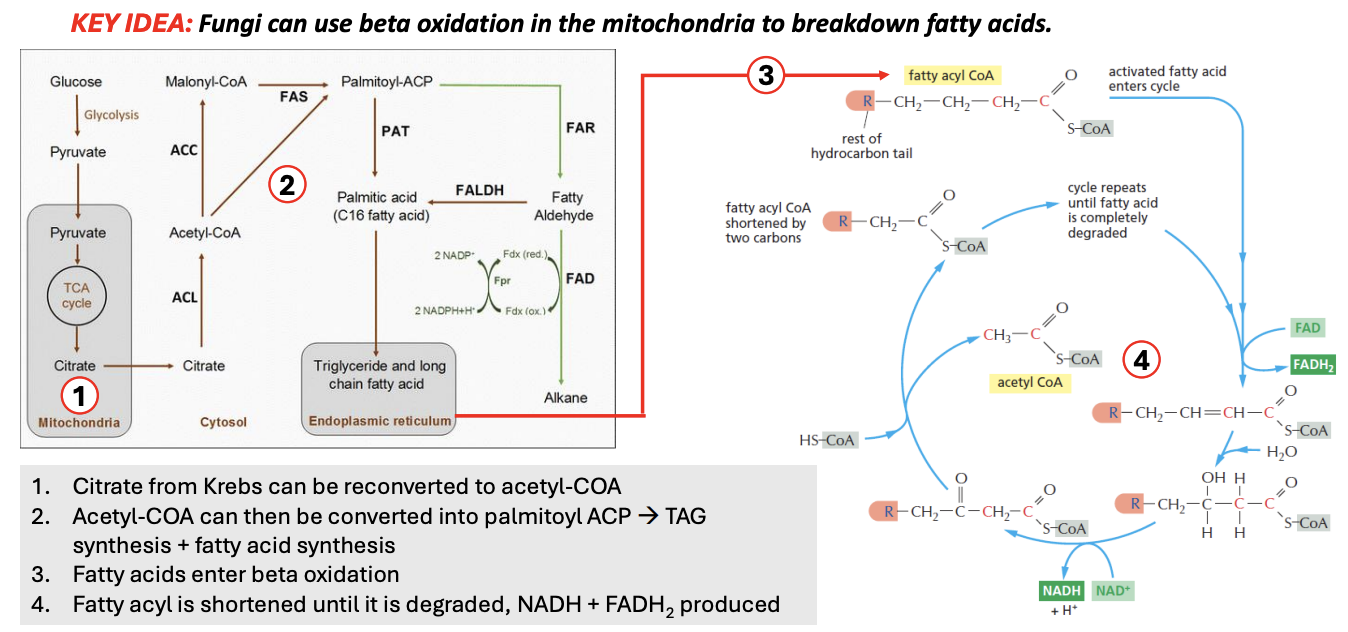
T/F: If the fungal cell needs to generate energy, then it’s most likely not going to export citrate, but if it needs to extend its hypha, then it will need to export some citrate to create phospholipids of plasma membrane
TRUE
Describe 2 circumstances where citrate would need to be exported out of mitochondria for fatty acid synthesis
If there is an absolute need to synthesize some kind of lipids, or
If there is an excess of materials and would want to store excess energy in the form of fat

_ are critical for fungi, as they use these for wns walls, nucleotides, and storage compounds; produced using 2 carbon precursors (acetate)
Sugars

Sugars are critical for fungi, as they use these as for _; produced using _
wns walls, nucleotides, storage compounds
2-carbon precursors → acetate
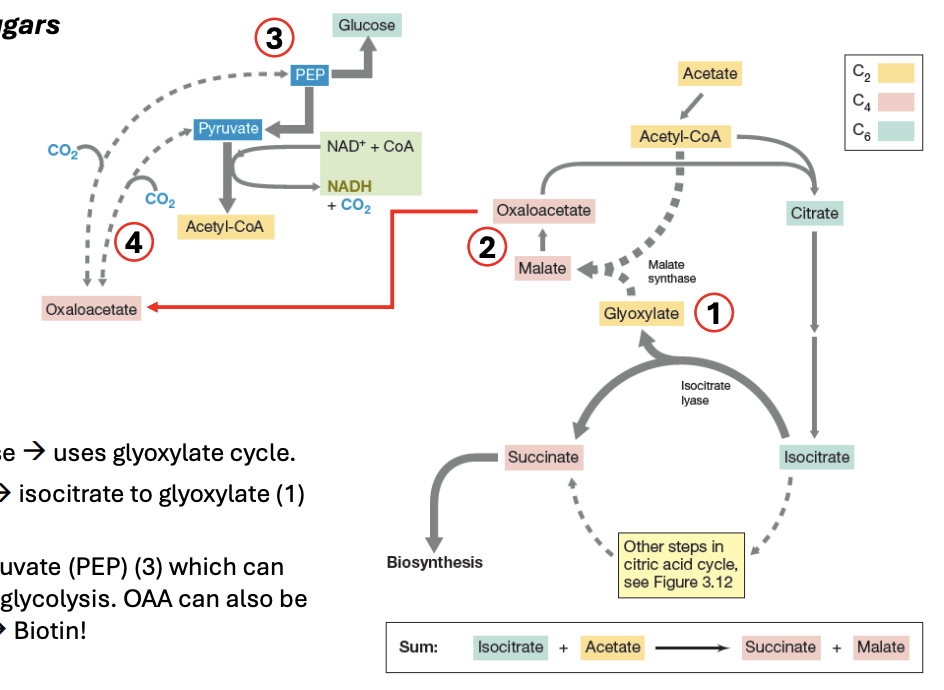
_ creates glucose via glyoxylate cycle
Gluconeogenesis
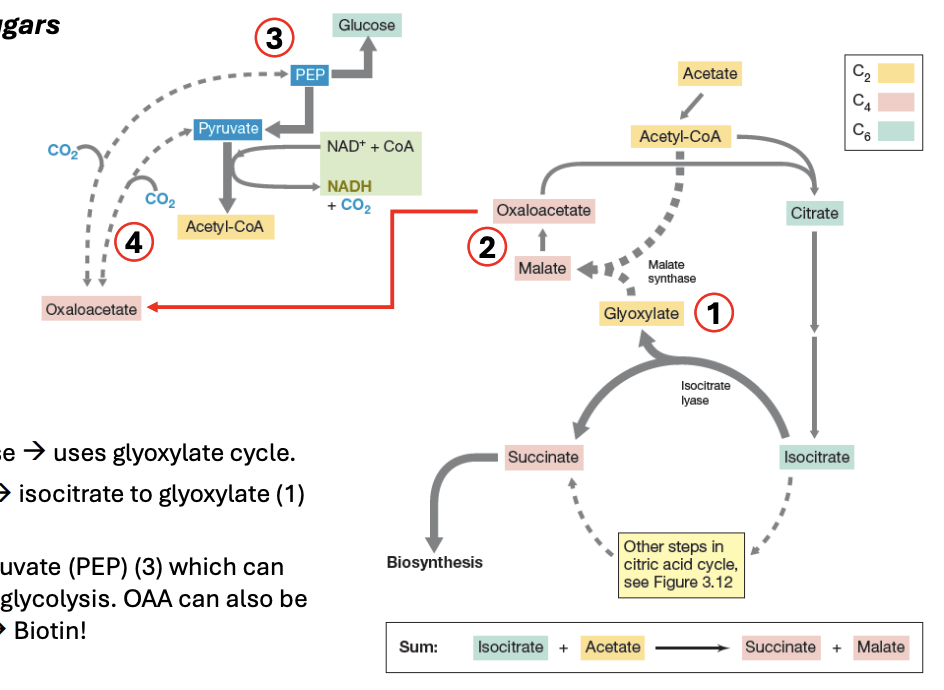
_ is a shortcut to Krebs, where isocitrate is converted to glyoxylate (2C), which, in turn, is converted to malate, then to oxaloacetate
OAA can be converted to PEP, which can then be used to create glucose via reverse glycolysis
By adding CO2 + pyruvate → OAA
Glyoxylate cycle
_ can be converted to PEP, which can then be used to create glucose via reverse glycolysis
OAA (oxaloacetate)

In glyoxylate cycle, OAA can be made by _
adding CO2 to pyruvate

T/F: Glyoxylate cycle is a sugar synthesis pathway
TRUE
(4C) OAA produced from shortcut glyoxylate cycle can be converted back to (3C) pyruvate → (6C) glucose
Explain the importance of glyoxylate cycle in fungi
Fungi use glyoxylate pathway to grow on acetate or fatty acids by bypassing the CO2-releasing steps of TCA, thus allowing carbon conservation for biosynthesis
It allows fungi to generate sugar from FA when carbohydrates are not available in their environment
Mammals lack this pathway, hence we cannot convert fats into glucose

Explain glyoxylate cycle
Acetyl CoA → TCA
6C citrate → 6C isocitrate
6C isocitrate → 2C glyoxylate + 4C succinate (biosynthesis)
2C glyoxylate + 2C acetyl CoA → 4C malate
4C malate → 4C OAA
4C OAA can be used to produce glucose via reverse glycolysis
4C OAA → (-CO2) 3C Pyruvate
3C pyruvate → 6C glucose
Or 4C OAA can be made by CO2 + (3C) pyruvate

During glyoxylate cycle, gluconeogenesis (glucose production) may occur by removing _ to OAA, producing either pyruvate or PEP
1 CO2
T/F: Glyoxylate is a four-carbon intermediate produced from isocitrate
FALSE
Glyoxylate is a two-carbon (2C) compound, while the other product, succinate, is four-carbon (4C)
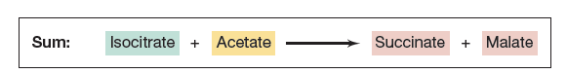
T/F: Fungi and bacteria rely on the glyoxylate cycle to survive in carbon-poor environments
TRUE
It allows them to use acetate or fatty acids as carbon sources
Fungi derive both chitin and chitosan from _
modification of glucose molecules
Explain composition/structure of chitin & chitosan
Chitin = 2 N-acetylglucosamine (NH=O)
Chitosan = 1 N-acetylglucosamine + 1 Glucosamine (NH2)

Explain chitin & chitosan biosynthesis
Glycogen → Glu-1-P → Glu-6-P
Trehalose → Glu → Glu-6-P
Glu-6-Pho → F6P → Glucosamine-6-P, converting glutamine → glutamic acid
Glucosamine-6-P → (Acetyl-CoA > CoA) N-acetylglucosamine-6-P
Glucosamine = precursor to N-acetylglucosamine
N-acetylglucosamine-6-P → N-a-1-p
Chitin (2 N-acetylglucosamine)
Chitosan (1 N-acetyl + 1 glucosamine)

Explain connected paths to EMP (Glycosis) & TCA
fpbg cl
Fermentation = to regenerate NAD+ for ATP production
Lactose & ethanol
Pentose Phosphate Pathway = regenerate active GSH for neutralizing ROS; produce sugar backbones for nucleotides, coenzymes, DNA, RNA; regenerate G3P, F6P that can re-enter EMP
Biosynthesis + energy production
Beta-oxidation = FAs converted into acetyl CoA for energy
Glyoxylation = use weird FAs/acetate to produce sugars (OAA > pyruvate > PEP > glucose)
Biosynthesis + energy production
Chitin & chitosan biosynthesis
Lysine biosynthesis
T/F: Chitosan is a deacetylated version of chitin with variable fraction of glucosamine residues
TRUE
Fungi synthesize the essential AA lysine via _
alpha-aminoadipate (AAA) pathway
_ are needed by fungi to synthesize proteins required for mcps mycelial growth, conidiation, stress resistance, and predacious abilities (e.g., nematode-trapping fungi)
Lysine
Lysine is needed by fungi to synthesize many proteins required for _
mcps
Mycelial growth
Conidiation
Stress
Predacious abilities (e.g., nematode-trapping fungi)